השם של חומצות שומן אלו נובע ממיקום הקשר הכפול בין אטומי הפחמן המרכיבים את המולקולה השומנית.
לשם השוואה, באומגה 3 הקשר הכפול הראשון נמצא בין אטום הפחמן השלישי והרביעי משייר המתיל ובאומגה 6 הקשר הכפול הראשון נמצא בין אטום הפחמן השישי והשביעי משייר המתיל.
הקשרים הכפולים מקנים לחומצות השומן הרב בלתי רוויות את התכונה הנוזלית (למשל שמן סויה) ואת יכולת החדירות.
בהיותה של מעטפת התא מורכבת גם מחומצות שומן אלו, הן מסייעות בויסות הכניסה של חומרים מזיקים לתא ומאפשרות כניסת חומרים חיוניים1.
לעומתן, חומצות השומן הרוויות מצויות במצב מוצק (למשל שמן דקלים או מרגרינה).
מבין חומצות השומן אומגה 3 נמנות:
-
חומצה אלפא לינולנית (ALA-(18:3 ω3)- Alpha Linolenic Acid) המכילה 18 פחמנים. היא היחידה המוגדרת כחיונית לאדם, כלומר לא ידוע על אנזימים המסוגלים לייצרה. מקורה מהצומח, למשל: זרעי פשתן, צ'יה, מרווה מרושתת, תרד, עלי רגלת הגינה, אגוזים (במיוחד מלך), שמן קנולה. הגוף יכול לאגור חלק מה-ALA שאנו צורכים ברקמת השומן, ולהשתמש בה כמקור אנרגיה וליצירת חומצות שומן אחרות.
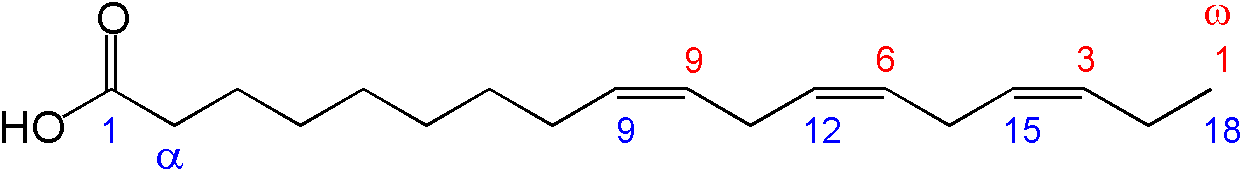
- חומצה איקוסאפנטאנואית (EPA-(20:5 ω3) Eicosapentaenoic Acid) המכילה 20 פחמנים. גוף האדם מסוגל לייצרה ממולקולת ALA על-ידי האנזימים Desaturase δ5 ו- Elongase. מקורה בעיקר מהחי: דגי ים שגדלו במים עמוקים ודגי ים צפוני הניזונים מאצות ים ופלנקטון (למשל סרדינים, סלמון, הרינג (מטיאס), טונה, מקרל, פורל, הליבוט, סול), בשר חיות שגדלו ואכלו בסביבה טבעית, אך גם אצות מסוימות.
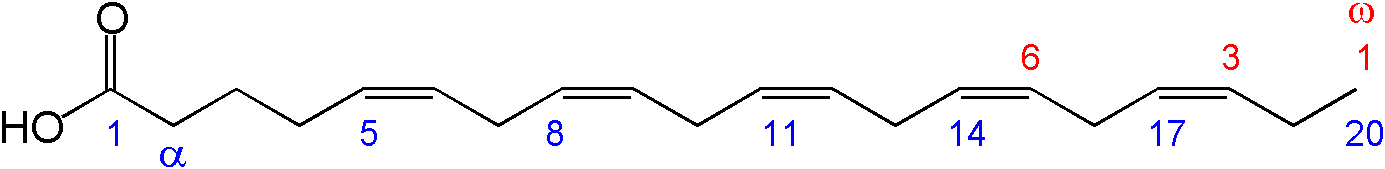
- חומצה דוקוסאהקסאנואית (DHA-(22:6 ω3) Docosahexaenoic Acid) המכילה 22 פחמנים. מקורה מהחי כמו EPA, ואותה גוף האדם מסוגל לייצר על-ידי הארכת EPA על-ידי האנזימים Desaturase δ6 ו- Elongase. היא חומצת השומן השכיחה ביותר במוח מבין חומצות השומן ארוכות השרשרת והבלתי רוויות. לכן היא בעלת תפקיד חשוב בהתפתחות הקוגניטיבית והמנטלית בעובר ובשלבי ההתפתחות המוקדמים לאחר הלידה.
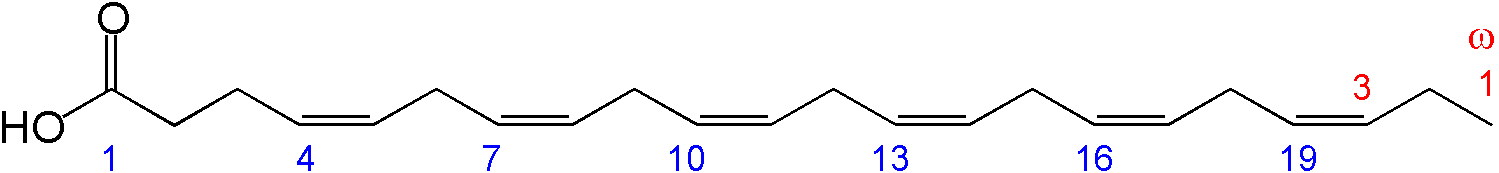
מבין חומצות השומן אומגה 6, החיונית לאדם היא החומצה האלפא לינולנית (LA), אותה הגוף מסוגל להאריך לחומצה אראכידונית (ARA).
הארכה זו דורשת את אותם האנזימים הנדרשים להארכת ALA ל- EPA, DHA.
מכאן שיש עיכוב תחרותי ביניהן, כלומר ככל שרמת האומגה 6 גבוהה יותר, פוחת ייצור ה- EPA וה-DHA.
מצד שני, כשרמת ה- ALA נמוכה, האנזימים "פנויים" ויכולים להמיר יותר LA ל-ARA.
בנוסף, תהליך ההמרה של ALA לחומצות הארוכות יותר מתרחש מעט מאוד בתרחיש נורמלי (ובמיוחד נכון הדבר לגבי ההארכה הארוכה יותר ל-DHA).
מחקרים מראים יעילות של עד 20% ל- EPA ועד 5% ל- DHA (כתלות ברמות תקינות של מגנזיום, אבץ וטסטוסטרון ובגנים מסוימים)2.
בנוסף, בקרב חלקים מהאוכלוסייה (למשל מאובחני ADHD) קיימת שונות גנטית אשר ככל הנראה גורמת לאחוזי המרה נמוכים יותר לעומת שאר האוכלוסייה3.
חשוב לציין כי עדיין לא ידוע מהם אחוזי ההמרה הדרושים לגוף וכי גם באוכלוסייה שאינה סובלת מהפגם הגנטי האמור, ההטמעה של DHA במוח היא 3.8±1.7 מיליגרם ליום בלבד, בעוד שבתרחישים מיוחדים כמו הריון נצפתה המרה של 9% ל-DHA משום צורך מוגבר לעובר4,5.
חומצות שומן מסוג אומגה 3 ואומגה 6 חיוניות למבנה של קרומי התאים אשר עשוי מפוספוליפידים המכילים חומצות שומן שונות. תפקוד הפוספוליפידים בממברנה תלוי בהרכב של חומצות השומן הנצרכות ומשפיע על החדירות והתקשורת של התא עם סביבתו (למשל כניסה ויציאה של חומרים שונים, העברת מסרים ומבנה קולטנים ויכולת היצמדות ההורמונים אליהם).
פירוק חומצות השומן החיוניות נעשה באמצעות אנזימים ממשפחת הפוספוליפאזות.
חומצות השומן EPA ו-ARA, המתפרקות מהפוספוליפידים על-ידי האנזים פוספוליפאז A2 יוצאות אל המרווח הבינתאי, מהוות חומרי מוצא ליצירת איקוסנואידים. אלו מוליקולות דמויות הורמונים מקומיים, המשפיעים על סביבתם הקרובה (זו השפעה פרא-קרינית).
מבנה האיקוסנואידים ותכונותיהם נקבעים על ידי סוג חומצות השומן מהן נוצרו (אומגה 3 או אומגה 6) והאנזימים שפועלים עליהן.
קיימים שני מסלולים אנזימטיים אליהם מגיעות חומצות השומן המשתחררות מהפוספוליפידים:
-
מסלול הציקלואוקסיגנאזה (COX) - מסלול זה מתאפשר בעזרת קבוצת אנזימים המכונה Cyclooxygenases COX ובו נוצרים איקוסנואידים מסוג פרוסטגלנדינים המשפיעים על כיווץ שרירים חלקים וכלי דם, טרומבקסנים המשפיעים על היצמדות טסיות דם ופרוסטציקלינים המונעים היצמדות טסיות, ממיסים קרישי דם ומרחיבים כלי דם.
-
מסלול ה-5 ליפואוקסיגינאזה (LOX) - מסלול זה נעשה בעזרת קבוצת האנזימים המכונים LOX Lypooxygenases ובו נוצרים איקוסנואידים מסוג לויקוטריאנים המשפיעים בעיקר על פעילות של מערכות העצבים והחיסון.
יציאה של EPA מהפוספוליפידים של התא גורמת לייצור של איקוסנואידים (PGE3, TX3, LTB5) אשר ממתנים תהליכי דלקת, מסייעים לתפקוד תקין של מערכות החיסון והעצבים ומונעים היצמדות טסיות דם.
לעומת זאת, יציאה של ARA גורמת לייצורם של איקוסנואידים (PGE2, TX2, LTB4) אשר מגבירים כאב, מעודדים תהליכי דלקת, מגבירים היצמדות טסיות דם, גורמים לכיווץ מוגבר של כלי הדם ופוגעים בתפקוד התקין של מערכות החיסון והעצבים.
עקב התחרות האנזימתית על ההמרה ל- EPA, DHA ו-ARA ומשום תהליכי הדלקת המוגברים שעלולים להופיע בנוכחות גבוהה של ARA, היחס הרצוי בין אומגה 3 לאומגה 6 עומד על 1:4 לטובת אומגה 6 ואף פחות מכך, בעוד שבתזונת העולם המערבי היחס עומד על 1:10-20 ואף יותר מכך, לטובת אומגה 6.
יש לציין שדגים כמקור לאומגה 3 הוא בעייתי לנמנעים מאכילת דגים מסיבות שונות (לא אוהבים, חוששים מכספית, צמחונים/טבעונים ושיקולים אקולוגיים).
עקב השימוש המוגבר, מקורות של דגים נכחדים במהירות גבוהה, ולכן מקורות צמחיים העשירים ב-ALA, נפוצים יותר ויכולים להוות תחליף.
מקורות תזונתיים לחומצות שומן חיוניות מסוג אומגה 3:
המקורות הטובים ביותר לחומצות שומן חיוניות מסוג אומגה 3 הינם דגי מים קרים.
קיימים מספר מקורות צמחיים אשר מכילים את חומצת השומן ALA אך הפיכתה לחומצות שומן EPA ו- DHA הינה מוגבלת (5-10%), תלויה ברמות תקינות של מגנזיום, אבץ וטסטוסטרון ובגנים מסוימים ולכן, בדרך כלל אינה מספקת את הכמויות הנדרשות לגוף.
מקורות מן הצומח: זרעי/שמן פשתן, שמן/אגוזי מלך, אגוזים אחרים ושקדים, מרווה, תרד, אצות, עלי ריג'לה.
מקורות מן החי: בעיקר דגי ים (מי ים עמוקים, ים צפוני) כגון סרדינים, סלמון, הרינג (מטיאס), טונה, מקרל, פורל, הליבוט, סול.
בשר בקר אשר ניזון מעשבים גם כן עשוי להכיל כמויות מסוימות של אומגה 3. כמו כן, קיימים מזונות כגון ביצים ומוצרי חלב אשר מועשרים בחומצות שומן חיוניות מסוג אומגה 3.
תפקידן של חומצות שומן חיוניות מסוג אומגה 3:
תכונות נוספות של חומצות שומן חיוניות מסוג אומגה 3:
חומצות שומן חיוניות עלולות להיהרס בחום וחשיפה לאור ולכן חשוב לשמור תוספים של אומגה 3 במקום חשוך וקר.
גורמים לחוסר בחומצות שומן חיוניות מסוג אומגה 3:
הפרעות ותסמינים הנגרמים עקב חוסר בחומצות שומן חיוניות מסוג אומגה 3:
מחסור בחומצות שומן חיוניות עשוי להתבטא בהפרעות שונות במערכות בגוף ולכלול:
עודף של חומצות שומן חיוניות מסוג אומגה 3 (רעילות):
לא דווח על רעילות כתוצאה מנטילה מוגזמת של אומגה 3.
בעת נטילת תוסף של אומגה 3 עלולות להופיע תופעות לוואי כגון למידע השלם למנויים
מינון יומי מומלץ של אומגה 3 על פי ה- (RDA (Recommended Daily Allowance:
לפי מסמך העמדה להמלצות תזונתיות למניעת מחלות קרדיווסקולריות (האיגוד הקרדיולוגי בישראל, 2015),
תוספי אומגה 3 הוכרו כ"בטוחים בדרך-כלל״ (GRAS) על-ידי ה-FDA, במינונים יומיים של עד 3 גרם (EPA+DHA).
להלן מינוני RDA בהתאם לגיל ומין8, לפי המכון אמריקני לרפואה (IOM):
ילדים:
0-12 חודשים: 0.5 גרם ביום.
1-3 שנים: 0.7 גרם ביום.
4-8 שנים: 0.9 גרם ביום.
גברים:
9-13 שנים: 1.2 גרם ביום.
14 שנים ומעלה: 1.6 גרם ביום.
נשים:
9-13 שנים: 1.0 גרם ביום.
14 שנים ומעלה: 1.1 גרם ביום.
בהריון: 1.4 גרם ביום.
בהנקה: 1.3 גרם ביום.
טווח מינון לטיפול בחוסר חומצות שומן חיוניות מסוג אומגה 3:
טווח המינון הטיפולי של אומגה 3 משתנה בהתאם למצבו של המטופל.
לדוגמא למניעה או טיפול במחלות לב וכלי דם יש ליטול למידע השלם למנויים
אזהרות וצעדי מנע
נטילה לפני/במקביל להליכים כירורגיים
יש לנקוט זהירות בעת נטילה בסמוך/במקביל להליכים כירורגיים.
בספרות מובעים חששות לגבי נטילת אומגה 3 בסמיכות להליכים כירורגיים עקב למידע השלם למנויים
תגובות הדדיות עם תרופות / צמחי מרפא / תוספי תזונה
תרופות למחלת הסרְטן
כימותרפיה | התרופה Ibrutinib | מעכבי ארומטאז | רדיותרפיה
צמחי מרפא ותוספי תזונה
גִ'ינסנג קוריאני (Panax gineseng) | וִיטמין D
המידע על האינטראקציות זמין למנויי האתר בלבד. לרכישת מנוי לחצו כאן.
הריון והנקה
אומגה 3 נחשבת בטוחה לשימוש בהריון והנקה.
סקירה משנת 2008 בחנה את העדות המדעית לתפקידן של חומצות שומן רב בלתי רוויות ארוכות שרשרת וחומצות שומן מסוג DHA ו-ARA
בהזנת תינוקות ובהתפתחותם. על פי ארגונים כמו האיגוד העולמי לרפואת טרום לידה והאקדמיה לתזונה והמוסד לבריאות הילד,
על העובר והולד לקבל את חומצות השומן הארוכות בכמויות המספיקות לתמיכה אופטימלית בהתפתחות קוגניטיבית וויזואלית.
צריכה של שמנים עשירים באומגה 3 במהלך ההריון למידע השלם למנויים
מחקרים על אומגה 3:
כללי | השפעה על מערכת הלב וכלי הדם | מערכת העצבים, תפקוד קוגניטיבי ובריאות הנפש | עור | מאזן שומנים וסוכר | גידולים סרטניים | ראייה
מקורות:
מקורות כלליים
Stargrove M B, Treasure J, McKee D. L, Herb, Nutrient, and Drug Interactions, Elsevier, 2008. pp 783-806.
www.naturaldatabase.com – Omega 3. found at - http://naturaldatabase.therapeuticresearch.com/nd/Search.aspx?cs=NONMP&s=ND&pt=100&id=993&ds=&name=Omega+3+(FISH+OIL)&searchid=30853101
אודי בר, יפה שיר-רז, "המדריך הישראלי השלם לתוספי תזונה", כתר ספרים, 2005
MedPortal פורטל בריאות ורפואה
https://www.medportal.co.il/%D7%97%D7%95%D7%9E%D7%A6%D7%95%D7%AA-%D7%A9%D7%95%D7%9E%D7%9F-%D7%90%D7%95%D7%9E%D7%92%D7%94-3-%D7%9B%D7%9C-%D7%9E%D7%94-%D7%A9%D7%97%D7%A9%D7%95%D7%91-%D7%9C%D7%93%D7%A2%D7%AA-%D7%A2%D7%9C-%D7%94/
www. Naturalstandard.com – Omega 3. found at - http://naturalstandard.com/databases/herbssupplements/fishoil.asp
מוריי מייקל ט., פיז'ורנו ג'וזף א., "אנציקלופדיה לרפואה טבעית", אור-עם, 1995
U.S. Institutes Of Health – Office Of Dietary Supplements – RDA tables. found at - http://www.nlm.nih.gov/medlineplus/calcium.html
.National Academy of Sciences. Institute of Medicine. Food and Nutrition Board
DRI table for DRI tables for recommended dietary allowances (RDA). found at - http://www.iom.edu/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx
Patrick Holford, "Special Report: Supplements – Optimum Daily Allowances". found at - http://www.patrickholford.com/index.php/advice/betterhealtharticle/138
מקורות פרטניים
- Mori TA. Omega-3 fatty acids and cardiovascular disease: epidemiology and effects on cardiometabolic risk factors. Food Funct. 2014 Sep;5(9):2004-19. https://pubmed.ncbi.nlm.nih.gov/25062404/
- Mori TA. Omega-3 fatty acids and hypertension in humans. Clin Exp Pharmacol Physiol. 2006 Sep;33(9):842-6. https://pubmed.ncbi.nlm.nih.gov/16922818/
- Cabo J, Alonso R, Mata P. Omega-3 fatty acids and blood pressure. Br J Nutr. 2012 Jun;107 Suppl 2:S195-200. https://pubmed.ncbi.nlm.nih.gov/22591893/
- Appel LJ, Miller ER 3rd, Seidler AJ, Whelton PK. Does supplementation of diet with 'fish oil' reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med. 1993 Jun 28;153(12):1429-38. https://pubmed.ncbi.nlm.nih.gov/8141868/
- Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002 Aug;20(8):1493-9. https://pubmed.ncbi.nlm.nih.gov/12172309/
- Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993 Aug;88(2):523-33. https://pubmed.ncbi.nlm.nih.gov/8339414/
- Lungershausen YK, Abbey M, Nestel PJ, Howe PR. Reduction of blood pressure and plasma triglycerides by omega-3 fatty acids in treated hypertensives. J Hypertens. 1994 Sep;12(9):1041-5. https://pubmed.ncbi.nlm.nih.gov/7852747/
- Singer P, Melzer S, Goschel M, Augustin S. Fish oil amplifies the effect of propranolol in mild essential hypertension. Hypertension. 1990 Dec;16(6):682-91. https://pubmed.ncbi.nlm.nih.gov/2147175/
- Gray DR, Gozzip CG, Eastham JH, Kashyap ML. Fish oil as an adjuvant in the treatment of hypertension. Pharmacotherapy. 1996 Mar-Apr;16(2):295-300. https://pubmed.ncbi.nlm.nih.gov/8820475/
- Howe PR, Lungershausen YK, Cobiac L, et al. Effect of sodium restriction and fish oil supplementation on BP and thrombotic risk factors in patients treated with ACE inhibitors. J Hum Hypertens. 1994 Jan;8(1):43-9. https://pubmed.ncbi.nlm.nih.gov/8151606/
- Woodman RJ, Mori TA, Burke V, et al. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis. 2003 Jan;166(1):85-93. https://pubmed.ncbi.nlm.nih.gov/12482554/
- Lichtenstein AH. Remarks on clinical data concerning dietary supplements that affect antithrombotic activity. Thromb Res 2005;117:71-3. https://pubmed.ncbi.nlm.nih.gov/15951007/
- Carr JA. Role of Fish Oil in Post-Cardiotomy Bleeding: A Summary of the Basic Science and Clinical Trials. Ann Thorac Surg. 2018 May;105(5):1563-1567. https://pubmed.ncbi.nlm.nih.gov/29627068/
- Harris WS. Expert opinion: omega-3 fatty acids and bleeding - cause for concern? Am J Cardiol 2007;99:44C-46C. https://pubmed.ncbi.nlm.nih.gov/17368278/
- Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol 2007;99:35C-43C. https://pubmed.ncbi.nlm.nih.gov/17368277/
- Lien EL. Toxicology and safety of DHA. Prostaglandins, leukotrienes, and essential fatty acids. Prostaglandins Leukot Essent Fatty Acids 2009;81:125-32. https://pubmed.ncbi.nlm.nih.gov/19501496/
- Wachira JK, Larson MK, Harris WS. n-3 Fatty acids affect haemostasis but do not increase the risk of bleeding: clinical observations and mechanistic insights. Br J Nutr. 2014 May;111(9):1652-62. https://pubmed.ncbi.nlm.nih.gov/24472372/
- Mousa SA. Antithrombotic effects of naturally derived products on coagulation and platelet function. Methods Mol Biol. 2010;663:229-40. https://pubmed.ncbi.nlm.nih.gov/20617421/
- Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012 Jan;3(1):1-7. https://pubmed.ncbi.nlm.nih.gov/22332096/
- Lovaza (omega-3-acid ethyl esters) [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021654s043lbl.pdf
- Vascepa (icosapent ethyl) [prescribing information]. Bridgewater, NJ: Amarin Pharma Inc.; December 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202057s035lbl.pdf
- Tavazzi L, Maggioni AP, Marchioli R, et al; Gissi-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223-30. https://pubmed.ncbi.nlm.nih.gov/18757090/
- Pryce R, Bernaitis N, Davey AK, et al. The Use of Fish Oil with Warfarin Does Not Significantly Affect either the International Normalised Ratio or Incidence of Adverse Events in Patients with Atrial Fibrillation and Deep Vein Thrombosis: A Retrospective Study. Nutrients. 2016 Sep 20;8(9). pii: nu8090578. https://pubmed.ncbi.nlm.nih.gov/27657121
- Buckley MS, Goff AD, Knapp WE. Fish oil interaction with warfarin. Ann Pharmacother. 2004 Jan;38(1):50-2. https://pubmed.ncbi.nlm.nih.gov/14742793
- Jalili M, Dehpour AR. Extremely prolonged INR associated with warfarin in combination with both trazodone and omega-3 fatty acids. Arch Med Res 2007;38:901-4. https://pubmed.ncbi.nlm.nih.gov/17923275/
- McClasky EM, Landrum Michalets E. Subdural hematoma after a fall in an elderly patient taking high-dose omega-3 fatty acids with warfarin and aspirin: case report and review of the literature. Pharmacotherapy 2007;27:152-60. https://pubmed.ncbi.nlm.nih.gov/17192169/
- Offman E, Davidson M, Nilsson C. No Effect of Omega-3 Carboxylic Acids on Pharmacokinetics/Pharmacodynamics of Warfarin or on Platelet Function When Co-administered with Acetylsalicylic Acid: Results of Two Phase I Studies in Healthy Volunteers. Am J Cardiovasc Drugs. 2017 Jun;17(3):251-260. https://pubmed.ncbi.nlm.nih.gov/28197979/
- Watson P, Joy P, Nkonde C, Hessen S, Karalis D. Comparison of bleeding complications with omega-3 fatty acids and aspirin and clopidogrel versus aspirin and clopidogrel in patients with cardiovascular disease. Am J Cardiol 2009;104:1052-4. https://pubmed.ncbi.nlm.nih.gov/19801023/
- Thorngren M, Gustafson A. Effects of 11-week increases in dietary eicosapentaenoic acid on bleeding time, lipids, and platelet aggregation. Lancet. 1981;2(8257):1190-1193. https://pubmed.ncbi.nlm.nih.gov/6118628/
- Serebruany VL, Miller M, Pokov AN, et al. Early impact of prescription Omega-3 fatty acids on platelet biomarkers in patients with coronary artery disease and hypertriglyceridemia. Cardiology. 2011;118(3):187-194. https://pubmed.ncbi.nlm.nih.gov/21701167/
- Iacoviello L, Amore C, De Curtis A, et al. Modulation of fibrinolytic response to venous occlusion in humans by a combination of low-dose aspirin and n-3 polyunsaturated fatty acids. Arterioscler Thromb. 1992 Oct;12(10):1191-7. https://pubmed.ncbi.nlm.nih.gov/1390591/
- Leaf A, Jorgensen MB, Jacobs AK, et al. Do fish oils prevent restenosis after coronary angioplasty? Circulation 1994;90:2248-57. https://pubmed.ncbi.nlm.nih.gov/7955181/
- Larson MK, Ashmore JH, Harris KA, et al. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb Haemost 2008;100:634-41. https://pubmed.ncbi.nlm.nih.gov/18841286/
- Lev EI, Solodky A, Harel N, et al. Treatment of aspirin-resistant patients with omega-3 fatty acids versus aspirin dose escalation. J Am Coll Cardiol. 2010;55:114–21. https://pubmed.ncbi.nlm.nih.gov/20117379/
- Gajos G, Rostoff P, Undas A, Piwowarska W. Effects of polyunsaturated omega-3 fatty acids on responsiveness to dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: the OMEGA-PCI (OMEGA-3 fatty acids after pci to modify responsiveness to dual antiplatelet therapy) study. J Am Coll Cardiol. 2010 Apr 20;55(16):1671-8. https://pubmed.ncbi.nlm.nih.gov/20394870
- Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Effects of eicosapentaenoic acid intake on plasma fibrinolytic and coagulation activity by using physical load in the young. Nutrition. 2000;16(1):11-14. https://pubmed.ncbi.nlm.nih.gov/10674228/
- Nilsen DW, Almdahl SM, Svensson B, et al. Lipopolysaccharide induced monocyte thromboplastin synthesis and coagulation responses in patients undergoing coronary bypass surgery after preoperative supplementation with n-3 fatty acids. Thromb Haemost. 1993;70(6):900-902. https://pubmed.ncbi.nlm.nih.gov/8165608/
- Nelson GJ, Schmidt PS, Bartolini GL, et al. The effect of dietary docosahexaenoic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids. 1997;32(11):1129-1136. https://pubmed.ncbi.nlm.nih.gov/9397397/
- Tomer A, Kasey S, Connor WE, Clark S, Harker LA, Eckman JR. Reduction of pain episodes and prothrombotic activity in sickle cell disease by dietary n-3 fatty acids. Thromb Haemost. 2001;85(6):966-974. https://pubmed.ncbi.nlm.nih.gov/11434703/
- Vanschoonbeek K, Feijge MA, Paquay M, et al. Variable hypocoagulant effect of fish oil intake in humans: modulation of fibrinogen level and thrombin generation. Arterioscler Thromb Vasc Biol. 2004;24(9):1734-1740. https://pubmed.ncbi.nlm.nih.gov/15217806/
- Shimizu H, Ohtani K, Tanaka Y, et al. Increased plasma thrombin-antithrombin III complex levels in non-insulin dependent diabetic patients with albuminuria are reduced by ethyl icosapentatenoate. Thromb Haemost. 1995;74(5):1231-1234. https://pubmed.ncbi.nlm.nih.gov/8607100/
- Yoshimura T, Matsui K, Ito M, et al. Effects of highly purified eicosapentaenoic acid on plasma beta thromboglobulin level and vascular reactivity to angiotensin II. Artery. 1987;14(5):295-303. https://pubmed.ncbi.nlm.nih.gov/2821970/
- Hay CR, Durber AP, Saynor R. Effect of fish oil on platelet kinetics in patients with ischaemic heart disease. Lancet. 1982;1(8284):1269-1270. https://pubmed.ncbi.nlm.nih.gov/6123019/
- Radack K, Deck C, Huster G. The comparative effects of n-3 and n-6 polyunsaturated fatty acids on plasma fibrinogen levels: a controlled clinical trial in hypertriglyceridemic subjects. J Am Coll Nutr. 1990;9(4):352-357. https://pubmed.ncbi.nlm.nih.gov/2212394/
- Sanders TA, Vickers M, Haines AP. Effect on blood lipids and haemostasis of a supplement of cod-liver oil, rich in eicosapentaenoic and docosahexaenoic acids, in healthy young men. Clin Sci (Lond). 1981;61(3):317-324. https://pubmed.ncbi.nlm.nih.gov/6266735/
- McEwen BJ, Morel-Kopp MC, Chen W, et al. Effects of omega-3 polyunsaturated fatty acids on platelet function in healthy subjects and subjects with cardiovascular disease. Semin Thromb Hemost. 2013 Feb;39(1):25-32. https://pubmed.ncbi.nlm.nih.gov/23329646
- Kepler CK, Huang RC, Meredith D, et al. Omega-3 and fish oil supplements do not cause increased bleeding during spinal decompression surgery. J Spinal Disord Tech. 2012 May;25(3):129-32. https://pubmed.ncbi.nlm.nih.gov/21423055/
- Freese R, Mutanen M. Alpha-linolenic acid and marine long-chain n-3 fatty acids differ only slightly in their effects on hemostatic factors in healthy subjects. Am J Clin Nutr. 1997;66(3):591-598. https://pubmed.ncbi.nlm.nih.gov/9280178/
- Goodnight SH Jr, Harris WS, Connor WE: The effects of dietary omega 3 fatty acids on platelet composition and function in man: a prospective, controlled study. Blood. 1981 Nov;58(5):880-5. https://pubmed.ncbi.nlm.nih.gov/7295999
- Heller AR, Fischer S, Rossel T, Geiger S, Siegert G, Ragaller M, et al. Impact of n-3 fatty acid supplemented parenteral nutrition on haemostasis patterns after major abdominal surgery. Br J Nutr 2002;87(Suppl 1):S95-S101. https://pubmed.ncbi.nlm.nih.gov/11895160/
- Terano T, Hirai A, Hamazaki T, et al. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis. 1983 Mar;46(3):321-31. https://pubmed.ncbi.nlm.nih.gov/6303363/
- Celik S, Doesch A, Erbel C, et al. Beneficial effect of omega-3 fatty acids on sirolimus- or everolimus-induced hypertriglyceridemia in heart transplant recipients. Transplantation. 2008 Jul 27;86(2):245-50. https://pubmed.ncbi.nlm.nih.gov/18645486/
- Cortinovis M, Gotti E, Remuzzi G, et al. Omega-3 polyunsaturated fatty acids affect sirolimus exposure in kidney transplant recipients on calcineurin inhibitor-free regimen. Transplantation. 2010;89(1):126-7. https://pubmed.ncbi.nlm.nih.gov/20061930/
- Robertsen I, Åsberg A, Jenssen TG, et al. Increased systemic exposure of once daily tacrolimus in renal transplant recipients on marine omega-3 fatty acid supplementation. Transpl Int. 2021. https://pubmed.ncbi.nlm.nih.gov/33991364/
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991 Sep;54(3):438-63. https://pubmed.ncbi.nlm.nih.gov/1908631/
- De Caterina R. Omega 3 fatty acids in renal diseases. World Rev Nutr Diet. 1994;76:137-42. https://pubmed.ncbi.nlm.nih.gov/7856224/
- Donadio JV Jr. Omega-3 polyunsaturated fatty acids: a potential new treatment of immune renal disease. Mayo Clin Proc. 1991 Oct;66(10):1018-28. https://pubmed.ncbi.nlm.nih.gov/1921484/
- Donadio JV. n-3 Fatty acids and their role in nephrologic practice. Curr Opin Nephrol Hypertens. 2001 Sep;10(5):639-42. https://pubmed.ncbi.nlm.nih.gov/11496058/
- Stoof TJ, Korstanje MJ, Bilo HJ, et al. Does fish oil protect renal function in cyclosporin-treated psoriasis patients? J Intern Med. 1989 Dec;226(6):437-41. https://pubmed.ncbi.nlm.nih.gov/2489230/
- Urakaze M, Hamazaki T, Kashiwabara H, Omori K, Fischer S, Yano S, Kumagai A. Favorable effects of fish oil concentrate on risk factors for thrombosis in renal allograft recipients. Nephron. 1989;53(2):102-9. https://pubmed.ncbi.nlm.nih.gov/2812166/
- Homan van der Heide JJ, Bilo HJ, Tegzess AM, Donker AJ. The effects of dietary supplementation with fish oil on renal function in cyclosporine-treated renal transplant recipients. Transplantation. 1990 Mar;49(3):523-7. https://pubmed.ncbi.nlm.nih.gov/2316014/
- Sweny P, Wheeler DC, Lui SF, Amin NS, et al. Dietary fish oil supplements preserve renal function in renal transplant recipients with chronic vascular rejection. Nephrol Dial Transplant. 1989;4(12):1070-5. https://pubmed.ncbi.nlm.nih.gov/2517328/
- Homan van der Heide JJ, Bilo HJ, Donker AJ, et al. Dietary supplementation with fish oil modifies renal reserve filtration capacity in postoperative, cyclosporin A-treated renal transplant recipients. Transpl Int. 1990 Oct;3(3):171-5. https://pubmed.ncbi.nlm.nih.gov/2271089/
- Ventura HO, Milani RV, Lavie CJ, et al. Cyclosporine-induced hypertension. Efficacy of omega-3 fatty acids in patients after cardiac transplantation. Circulation. 1993 Nov;88(5 Pt 2):II281-5. https://pubmed.ncbi.nlm.nih.gov/8222166/
- Holm T, Andreassen AK, Aukrust P, et al. Omega-3 fatty acids improve blood pressure control and preserve renal function in hypertensive heart transplant recipients. Eur Heart J. 2001 Mar;22(5):428-36. https://pubmed.ncbi.nlm.nih.gov/11207085/
- Andreassen AK, Hartmann A, Offstad J, et al. Hypertension prophylaxis with omega-3 fatty acids in heart transplant recipients. J Am Coll Cardiol. 1997 May;29(6):1324-31. https://pubmed.ncbi.nlm.nih.gov/9137231/
- Sabry A, El-Husseini A, Sheashaa H, et al. Colchicine vs. omega-3 fatty acids for prevention of chronic cyclosporine nephrotoxicity in Sprague Dawley rats: an experimental animal model. Arch Med Res. 2006 Nov;37(8):933-40. https://pubmed.ncbi.nlm.nih.gov/17045107/
- Elzinga L, Kelley VE, Houghton DC, Bennett WM. Modification of experimental nephrotoxicity with fish oil as the vehicle for cyclosporine. Transplantation. 1987 Feb;43(2):271-4. https://pubmed.ncbi.nlm.nih.gov/3810835/
- Walker RJ, Lazzaro VA, Duggin GG, et al. Dietary eicosapentaenoic acid does not modify cyclosporin-induced inhibition of angiotensin II-stimulated prostaglandin synthesis in mesangial cells. Ren Fail. 1989;11(2-3):125-32. https://pubmed.ncbi.nlm.nih.gov/2623198/
- Sabry A, El-Dahshan K, El-Hussieni A. Prevention of chronic cyclosporine nephrotoxicity in Sprague Dawely rats: role of colchicine and omega-3-fatty acids. Int Urol Nephrol. 2007;39(1):271-3. https://pubmed.ncbi.nlm.nih.gov/17333531/
- Goksu Erol AY, Avcı G, Sevimli A, et al. The protective effects of omega 3 fatty acids and sesame oil against cyclosporine A-induced nephrotoxicity. Drug Chem Toxicol. 2013 Apr;36(2):241-8. https://pubmed.ncbi.nlm.nih.gov/22950701/
- Busnach G, Stragliotto E, Minetti E, et al. Effect of n-3 polyunsaturated fatty acids on cyclosporine pharmacokinetics in kidney graft recipients: a randomized placebo-controlled study. J Nephrol. 1998 Mar-Apr;11(2):87-93. https://pubmed.ncbi.nlm.nih.gov/9589380
- Brown TJ, Brainard J, Song F, et al; PUFAH Group. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019 Aug 21;366:l4697. https://pubmed.ncbi.nlm.nih.gov/31434641/
- Chen C, Yu X, Shao S. Effects of Omega-3 Fatty Acid Supplementation on Glucose Control and Lipid Levels in Type 2 Diabetes: A Meta-Analysis. PLoS One. 2015 Oct 2;10(10):e0139565. https://pubmed.ncbi.nlm.nih.gov/26431431/
- Gao C, Liu Y, Gan Y, et al. Effects of fish oil supplementation on glucose control and lipid levels among patients with type 2 diabetes mellitus: a Meta-analysis of randomized controlled trials. Lipids Health Dis. 2020 May 8;19(1):87. https://pubmed.ncbi.nlm.nih.gov/32384902/
- Flachs P, Rossmeisl M, Kopecky J. The effect of n-3 fatty acids on glucose homeostasis and insulin sensitivity. Physiol Res. 2014;63(Suppl 1):S93-118. https://pubmed.ncbi.nlm.nih.gov/24564669/
- Jialal I, Amess W, Kaur M. Management of hypertriglyceridemia in the diabetic patient. Curr Diab Rep. 2010 Aug;10(4):316-20. https://pubmed.ncbi.nlm.nih.gov/20532703/
- Ghadge AA, Kuvalekar AA. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: Novel move towards combination therapies. Diabetes Metab Syndr. 2017 Nov;11 Suppl 1:S5-S13. https://pubmed.ncbi.nlm.nih.gov/27578618/
- Alexopoulos AS, Qamar A, Hutchins K, et al. Triglycerides: Emerging Targets in Diabetes Care? Review of Moderate Hypertriglyceridemia in Diabetes. Curr Diab Rep. 2019 Feb 26;19(4):13. https://pubmed.ncbi.nlm.nih.gov/30806837/
- Hartweg J, Farmer AJ, Perera R, et al. Meta-analysis of the effects of n-3 polyunsaturated fatty acids on lipoproteins and other emerging lipid cardiovascular risk markers in patients with type 2 diabetes. Diabetologia. 2007;50:1593-1602. https://pubmed.ncbi.nlm.nih.gov/17541540/
- Yang S, Lin R, Si L, et al. Cod-liver oil improves metabolic indices and hs-CRP levels in gestational diabetes mellitus patients: A double-blind randomized controlled trial. J Diabetes Res. 2019;2019:7074042. https://pubmed.ncbi.nlm.nih.gov/31956660/
- Woodman RJ, Mori TA, Burke V, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 2002;76:1007-15. https://pubmed.ncbi.nlm.nih.gov/12399272/
- Chauhan S, Kodali H, Noor J, et al. Role of Omega-3 Fatty Acids on Lipid Profile in Diabetic Dyslipidaemia: Single Blind, Randomised Clinical Trial. J Clin Diagn Res. 2017 Mar;11(3):OC13-OC16. https://pubmed.ncbi.nlm.nih.gov/28511427/
- Azadbakht L, Rouhani MH, Surkan PJ. Omega-3 fatty acids, insulin resistance and type 2 diabetes. J Res Med Sci. 2011 Oct;16(10):1259-60. https://pubmed.ncbi.nlm.nih.gov/22973318/
- Ramel A, Martinez A, Kiely M, et al. Beneficial effects of long-chain n-3 fatty acids included in an energy-restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia. 2008;51(7):1261–8. https://pubmed.ncbi.nlm.nih.gov/18491071/
- Jin S, Sha L, Dong J, et al. Effects of Nutritional Strategies on Glucose Homeostasis in Gestational Diabetes Mellitus: A Systematic Review and Network Meta-Analysis. J Diabetes Res. 2020 Feb 23;2020:6062478. https://pubmed.ncbi.nlm.nih.gov/32185236/
- Patti AM, Giglio RV, Papanas N, Rizzo M, Rizvi AA. Future perspectives of the pharmacological management of diabetic dyslipidemia. Expert Rev Clin Pharmacol. 2019 Feb;12(2):129-143. https://pubmed.ncbi.nlm.nih.gov/30644763/
- Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138-146. https://pubmed.ncbi.nlm.nih.gov/19202385/
- Ibrutinib FDA drug label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210563s000lbl.pdf
- Brinson BE, Miller S. Fish oil: what is the role in cardiovascular health? J Pharm Pract. 2012 Feb;25(1):69-74. https://pubmed.ncbi.nlm.nih.gov/21676848/
- Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012 May 31;1:26. https://pubmed.ncbi.nlm.nih.gov/22651380/
- Sethi A, Bajaj A, Khosla S, Arora RR. Statin Use Mitigate the Benefit of Omega-3 Fatty Acids Supplementation-A Meta-Regression of Randomized Trials. Am J Ther. 2016 May-Jun;23(3):e737-48. https://pubmed.ncbi.nlm.nih.gov/25036814/
- Bhatt DL, Steg PG, Miller M, et al.; REDUCE-IT Investigators. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019 Jan 3;380(1):11-22. https://pubmed.ncbi.nlm.nih.gov/30415628/
- Reddy KJ, Chowdhury S. Improving lipids with prescription icosapent ethyl after previous use of fish oil dietary supplements. Future Cardiol. 2016;12:261-268. https://pubmed.ncbi.nlm.nih.gov/27070379/
- Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110:984-992. https://pubmed.ncbi.nlm.nih.gov/22819432/
- Nelson SD, Munger MA. Icosapent ethyl for treatment of elevated triglyceride levels. Ann Pharmacother. 2013 Nov;47(11):1517-23. https://pubmed.ncbi.nlm.nih.gov/24259598/
- Nair AP, Darrow B. Lipid management in the geriatric patient. Endocrinol Metab Clin North Am. 2009 Mar;38(1):185-206. https://pubmed.ncbi.nlm.nih.gov/19217519/
- Cannon CP. Combination therapy in the management of mixed dyslipidaemia. J Intern Med. 2008 Apr;263(4):353-65. https://pubmed.ncbi.nlm.nih.gov/18324928/
- Vasudevan AR, Jones PH. Effective use of combination lipid therapy. Curr Cardiol Rep. 2005 Nov;7(6):471-9. https://pubmed.ncbi.nlm.nih.gov/16256018/
- Backes J, Anzalone D, Hilleman D, Catini J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016 Jul 22;15(1):118. https://pubmed.ncbi.nlm.nih.gov/27444154/
- Bird JK, Calder PC, Eggersdorfer M. The Role of n-3 Long Chain Polyunsaturated Fatty Acids in Cardiovascular Disease Prevention, and Interactions with Statins. Nutrients. 2018 Jun 15;10(6):775. https://pubmed.ncbi.nlm.nih.gov/29914111/
- Feingold KR. Triglyceride Lowering Drugs. 2021 Apr 1. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. https://pubmed.ncbi.nlm.nih.gov/28402615/
- Kar S. Omacor and omega-3 fatty acids for treatment of coronary artery disease and the pleiotropic effects. Am J Ther. 2014 Jan-Feb;21(1):56-66. https://pubmed.ncbi.nlm.nih.gov/21975796/
- Ito MK. Long-chain omega-3 fatty acids, fibrates and niacin as therapeutic options in the treatment of hypertriglyceridemia: a review of the literature. Atherosclerosis. 2015 Oct;242(2):647-56. https://pubmed.ncbi.nlm.nih.gov/26296750/
- Villalobos ME, Sánchez-Muniz FJ, Acín MT, et al. Similitudes, diferencias y agonismos en los efectos pleiotrópicos de las estatinas y los ácidos grasos omega-3 [Similarities, differences and agonisms of pleiotropic effects of statins and omega-3 fatty acids]. Nutr Hosp. 2010 Nov-Dec;25(6):889-909. Spanish. https://pubmed.ncbi.nlm.nih.gov/21519759/
- Mori TA. Dietary n-3 PUFA and CVD: a review of the evidence. Proc Nutr Soc. 2014 Feb;73(1):57-64. https://pubmed.ncbi.nlm.nih.gov/24119287/
- Farnier M. Safety review of combination drugs for hyperlipidemia. Expert Opin Drug Saf. 2011 May;10(3):363-71. https://pubmed.ncbi.nlm.nih.gov/21417957/
- Kostapanos MS, Milionis HJ, Elisaf MS. Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia. Am J Cardiovasc Drugs. 2010;10(1):11-28. https://pubmed.ncbi.nlm.nih.gov/20104931/
- Maki KC, Dicklin MR, Davidson MH, et al.; COMBination of prescription Omega-3 with Simvastatin (COMBOS) Investigators. Baseline lipoprotein lipids and low-density lipoprotein cholesterol response to prescription omega-3 acid ethyl ester added to Simvastatin therapy. Am J Cardiol. 2010 May 15;105(10):1409-12. https://pubmed.ncbi.nlm.nih.gov/20451686/
- Carrepeiro MM, Rogero MM, Bertolami MC, et al. Effect of n-3 fatty acids and statins on oxidative stress in statin-treated hypercholestorelemic and normocholesterolemic women. Atherosclerosis. 2011 Jul;217(1):171-8. https://pubmed.ncbi.nlm.nih.gov/21561620/
- Eussen SR, Geleijnse JM, Giltay EJ, et al. Effects of n-3 fatty acids on major cardiovascular events in statin users and non-users with a history of myocardial infarction. Eur Heart J. 2012 Jul;33(13):1582-8. https://pubmed.ncbi.nlm.nih.gov/22301766/
- Di Spirito M, Morelli G, Doyle RT, et al. Effect of omega-3-acid ethyl esters on steady-state plasma pharmacokinetics of atorvastatin in healthy adults. Expert Opin Pharmacother. 2008 Dec;9(17):2939-45. https://pubmed.ncbi.nlm.nih.gov/19006470/
- Gosai P, Liu J, Doyle RT, et al. Effect of omega-3-acid ethyl esters on the steady-state plasma pharmacokinetics of rosuvastatin in healthy adults. Expert Opin Pharmacother. 2008 Dec;9(17):2947-53. https://pubmed.ncbi.nlm.nih.gov/19006471/
- McKenney JM, Swearingen D, Di Spirito M, et al. Study of the pharmacokinetic interaction between simvastatin and prescription omega-3-acid ethyl esters. J Clin Pharmacol. 2006 Jul;46(7):785-91. https://pubmed.ncbi.nlm.nih.gov/16809804/
- Offman E, Davidson M, Nilsson C. Assessment of pharmacokinetic interaction between omega-3 carboxylic acids and the statins rosuvastatin and simvastatin: Results of 2 phase I studies in healthy volunteers. J Clin Lipidol. 2017 May- Jun;11(3):739-748. https://pubmed.ncbi.nlm.nih.gov/28506390/
- Roth EM, Bays HE, Forker AD, et al. Prescription omega-3 fatty acid as an adjunct to fenofibrate therapy in hypertriglyceridemic subjects. J Cardiovasc Pharmacol. 2009 Sep;54(3):196-203. https://pubmed.ncbi.nlm.nih.gov/19597368/
- Reiner Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol. 2017;14:401-411. https://pubmed.ncbi.nlm.nih.gov/28300080/
- Xenical FDA drug label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020766s026lbl.pdf
- Cruz-Hernandez C, Destaillats F, Thakkar SK, et al. Monoacylglycerol-enriched oil increases EPA/DHA delivery to circulatory system in humans with induced lipid malabsorption conditions. J Lipid Res. 2016 Dec;57(12):2208-2216. https://pubmed.ncbi.nlm.nih.gov/27707818/
- Cruz-Hernandez C, Oliveira M, Pescia G, et al. Lipase inhibitor orlistat decreases incorporation of eicosapentaenoic and docosahexaenoic acids in rat tissues. Nutr Res. 2010 Feb;30(2):134-40. https://pubmed.ncbi.nlm.nih.gov/20226999/
- Cruz-Hernandez C, Thakkar SK, Moulin J, et al. Benefits of structured and free monoacylglycerols to deliver eicosapentaenoic (EPA) in a model of lipid malabsorption. Nutrients. 2012 Nov 21;4(11):1781-93. https://pubmed.ncbi.nlm.nih.gov/23201848/
- Yao HT, Chang YW, Lan SJ, Chen CT, Hsu JT, Yeh TK. The inhibitory effect of polyunsaturated fatty acids on human CYP enzymes. Life Sci. 2006 Nov 25;79(26):2432-40. https://pubmed.ncbi.nlm.nih.gov/16978661/
- Hirunpanich V, Katagi J, Sethabouppha B, Sato H. Demonstration of docosahexaenoic acid as a bioavailability enhancer for CYP3A substrates: in vitro and in vivo evidence using cyclosporin in rats. Drug Metab Dispos. 2006 Feb;34(2):305-10. https://pubmed.ncbi.nlm.nih.gov/16299163/
- Lam CN, Watt AE, Isenring EA, et al. The effect of oral omega-3 polyunsaturated fatty acid supplementation on muscl0e maintenance and quality of life in patients with cancer: A systematic review and meta-analysis. Clin Nutr. 2021 Jun;40(6):3815-3826. https://pubmed.ncbi.nlm.nih.gov/34130028/
- Akita H, Takahashi H, Asukai K, et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: Prospective randomized control study. Clin Nutr ESPEN. 2019 Oct;33:148-153. https://pubmed.ncbi.nlm.nih.gov/31451252/
- Anoushirvani AA, Poorsaadat L, Aghabozorgi R, Kasravi M. Comparison of the Effects of Omega 3 and Vitamin E on Palcitaxel-Induced Peripheral Neuropathy. Open Access Maced J Med Sci. 2018 Oct 21;6(10):1857-1861. https://pubmed.ncbi.nlm.nih.gov/30455762/
- Aredes MA, da Camara AO, de Paula NS, et al. Efficacy of ω-3 supplementation on nutritional status, skeletal muscle, and chemoradiotherapy toxicity in cervical cancer patients: A randomized, triple-blind, clinical trial conducted in a middle-income country. Nutrition. 2019 Nov-Dec;67-68:110528. https://pubmed.ncbi.nlm.nih.gov/31445316/
- Bonatto SJ, Oliveira HH, Nunes EA, et al. Fish oil supplementation improves neutrophil function during cancer chemotherapy. Lipids. 2012 Apr;47(4):383-9. https://pubmed.ncbi.nlm.nih.gov/22160495/
- Camargo Cde Q, Mocellin MC, Pastore Silva Jde A, et al. Fish oil supplementation during chemotherapy increases posterior time to tumor progression in colorectal cancer. Nutr Cancer. 2016;68(1):70-6. https://pubmed.ncbi.nlm.nih.gov/26700096/
- Chagas TR, Borges DS, de Oliveira PF, et al. Oral fish oil positively influences nutritional-inflammatory risk in patients with haematological malignancies during chemotherapy with an impact on long-term survival: a randomised clinical trial. J Hum Nutr Diet. 2017 Dec;30(6):681-692. https://pubmed.ncbi.nlm.nih.gov/28374923/
- de la Rosa Oliva F, Meneses García A, Ruiz Calzada H, et al. Effects of omega-3 fatty acids supplementation on neoadjuvant chemotherapy-induced toxicity in patients with locally advanced breast cancer: a randomized, controlled, double-blinded clinical trial. Nutr Hosp. 2019 Aug 26;36(4):769-776. English. https://pubmed.ncbi.nlm.nih.gov/31192682/
- Esfahani A, Somi MH, Ayromlou H, et al. The effect of n-3 polyunsaturated fatty acids on incidence and severity of oxaliplatin induced peripheral neuropathy: a randomized controlled trial. Biomark Res. 2016 Jun 23;4:13. https://pubmed.ncbi.nlm.nih.gov/27340553/
- Fearon KC, Von Meyenfeldt MF, Moses AG, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003 Oct;52(10):1479-86. https://pubmed.ncbi.nlm.nih.gov/12970142/
- Finocchiaro C, Segre O, Fadda M, et al. Effect of n-3 fatty acids on patients with advanced lung cancer: a double-blind, placebo-controlled study. Br J Nutr. 2012 Jul;108(2):327-33. https://pubmed.ncbi.nlm.nih.gov/22114792/
- Ghoreishi Z, Esfahani A, Djazayeri A, et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer. 2012 Aug 15;12:355. https://pubmed.ncbi.nlm.nih.gov/22894640/
- Gogos CA, Ginopoulos P, Salsa B, et al. Dietary omega-3 polyunsaturated fatty acids plus Vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer. 1998 Jan 15;82(2):395-402. https://pubmed.ncbi.nlm.nih.gov/9445198/
- Guarcello M, Riso S, Buosi R, D'Andrea F. EPA-enriched oral nutritional support in patients with lung cancer: effects on nutritional status and quality of life. Nutr Ther Metabol 2007;25(1):25e30.
- Haidari F, Abiri B, Iravani M, et al. Randomized Study of the Effect of Vitamin D and Omega-3 Fatty Acids Cosupplementation as Adjuvant Chemotherapy on Inflammation and Nutritional Status in Colorectal Cancer Patients. J Diet Suppl. 2020;17(4):384-400. https://pubmed.ncbi.nlm.nih.gov/31106659/
- Hanai N, Terada H, Hirakawa H, et al. Prospective randomized investigation implementing immunonutritional therapy using a nutritional supplement with a high blend ratio of ω-3 fatty acids during the perioperative period for head and neck carcinomas. Jpn J Clin Oncol. 2018 Apr 1;48(4):356-361. https://pubmed.ncbi.nlm.nih.gov/29420749/
- Hossain T, Phillips BE, Doleman B, et al. A double-blind randomized controlled trial of the effects of eicosapentaenoic acid supplementation on muscle inflammation and physical function in patients undergoing colorectal cancer resection. Clin Nutr. 2020 Jul;39(7):2055-2061. https://pubmed.ncbi.nlm.nih.gov/31648815/
- Aoyama T, Yoshikawa T, Ida S, et al. Effects of perioperative Eicosapentaenoic acid-enriched oral nutritional supplement on lean body mass after total gastrectomy for gastric cancer. J Cancer. 2019 Jan 29;10(5):1070-1076. https://pubmed.ncbi.nlm.nih.gov/30854113/
- Ida S, Hiki N, Cho H, et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br J Surg. 2017 Mar;104(4):377-383. https://pubmed.ncbi.nlm.nih.gov/28072447/
- Lustberg MB, Orchard TS, Reinbolt R, et al. Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res Treat. 2018 Feb;167(3):709-718. https://pubmed.ncbi.nlm.nih.gov/29101597/
- Mocellin MC, Pastore e Silva Jde A, Camargo Cde Q, et al. Fish oil decreases C-reactive protein/albumin ratio improving nutritional prognosis and plasma fatty acid profile in colorectal cancer patients. Lipids. 2013 Sep;48(9):879-88. https://pubmed.ncbi.nlm.nih.gov/23888317/
- Mocellin MC, Camargo CdQ, Fabre MEdS, Trindade EBSdM. Fish oil effects on quality of life, body weight and free fat mass change in gastrointestinal cancer patients undergoing chemotherapy: a triple blind, randomized clinical trial. J Functional Foods 2017;31:113e22.
- Camargo CQ, Mocellin MC, Brunetta HS, et al. Fish oil decreases the severity of treatment-related adverse events in gastrointestinal cancer patients undergoing chemotherapy: a randomized, placebo-controlled, triple-blind clinical trial. Clin Nutr ESPEN 2019;31:61e70.
- Paixão EMDS, Oliveira ACM, Pizato N, et al. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naïve breast cancer patients: a randomized double-blind controlled trial. Nutr J. 2017 Oct 23;16(1):71. https://pubmed.ncbi.nlm.nih.gov/29061183/
- Roca-Rodríguez MM, García-Almeida JM, Lupiañez-Pérez Y, et al. Effect of a specific supplement enriched with n-3 polyunsaturated fatty acids on markers of inflammation, oxidative stress and metabolic status of ear, nose and throat cancer patients. Oncol Rep. 2014 Jan;31(1):405-14. https://pubmed.ncbi.nlm.nih.gov/24154820/
- Sánchez-Lara K, Turcott JG, Juárez-Hernández E, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr. 2014 Dec;33(6):1017-23. https://pubmed.ncbi.nlm.nih.gov/24746976/
- Silva Jde A, Trindade EB, Fabre ME, et al. Fish oil supplement alters markers of inflammatory and nutritional status in colorectal cancer patients. Nutr Cancer. 2012;64(2):267-73. https://pubmed.ncbi.nlm.nih.gov/22295891/
- Solís-Martínez O, Plasa-Carvalho V, Phillips-Sixtos G, et al. Effect of Eicosapentaenoic Acid on Body Composition and Inflammation Markers in Patients with Head and Neck Squamous Cell Cancer from a Public Hospital in Mexico. Nutr Cancer. 2018 May-Jun;70(4):663-670. https://pubmed.ncbi.nlm.nih.gov/29697274/
- Suzumura DN, Schleder JC, Appel MH, et al. Fish Oil Supplementation Enhances Pulmonary Strength and Endurance in Women Undergoing Chemotherapy. Nutr Cancer. 2016 Aug-Sep;68(6):935-42. https://pubmed.ncbi.nlm.nih.gov/27340931/
- Trabal J, Leyes P, Forga M, Maurel J. Potential usefulness of an EPA-enriched nutritional supplement on chemotherapy tolerability in cancer patients without overt malnutrition. Nutr Hosp. 2010 Sep-Oct;25(5):736-40. https://pubmed.ncbi.nlm.nih.gov/21336429/
- van der Meij BS, Langius JA, Smit EF, et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J Nutr. 2010 Oct;140(10):1774-80. https://pubmed.ncbi.nlm.nih.gov/20739445/
- van der Meij BS, Langius JA, Spreeuwenberg MD, et al. Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT. Eur J Clin Nutr. 2012 Mar;66(3):399-404. https://pubmed.ncbi.nlm.nih.gov/22234041/
- Elsadek AE, Maksoud YHA, Suliman HA, et al. Omega-3 supplementation in children with ADHD and intractable epilepsy. J Clin Neurosci. 2021 Dec;94:237-243. https://pubmed.ncbi.nlm.nih.gov/34863444/
- Appleton KM, Voyias PD, Sallis HM, et al. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 2021 Nov 24;11(11):CD004692. https://pubmed.ncbi.nlm.nih.gov/34817851/
- Bot M, Pouwer F, Assies J, et al. Eicosapentaenoic acid as an add-on to antidepressant medication for co-morbid major depression in patients with diabetes mellitus: a randomized, double-blind placebo-controlled study. J Affect Disord. 2010 Oct;126(1-2):282-6. https://pubmed.ncbi.nlm.nih.gov/20466431/
- Carney RM, Freedland KE, Rubin EH, et al. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA. 2009 Oct 21;302(15):1651-7. https://pubmed.ncbi.nlm.nih.gov/19843899/
- Carney RM, Freedland KE, Rubin EH, et al. A Randomized Placebo-Controlled Trial of Omega-3 and Sertraline in Depressed Patients With or at Risk for Coronary Heart Disease. J Clin Psychiatry. 2019 Jun 4;80(4):19m12742. https://pubmed.ncbi.nlm.nih.gov/31163106/
- Chang JP, Chang SS, Yang HT, et al. Omega-3 polyunsaturated fatty acids in cardiovascular diseases comorbid major depressive disorder - Results from a randomized controlled trial. Brain Behav Immun. 2020 Mar;85:14-20. https://pubmed.ncbi.nlm.nih.gov/30902738/
- Fiedorowicz JG, Hale N, Spector AA, Coryell WH. Neuroticism but not omega-3 fatty acid levels correlate with early responsiveness to escitalopram. Ann Clin Psychiatry. 2010 Aug;22(3):157-63. https://pubmed.ncbi.nlm.nih.gov/20680188/
- da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008 Dec;111(2-3):351-9. https://pubmed.ncbi.nlm.nih.gov/18485485/
- Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol. 2012 Feb;32(1):61-4. https://pubmed.ncbi.nlm.nih.gov/22198441/
- Gharekhani A, Khatami MR, Dashti-Khavidaki S, et al. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: a randomized, placebo-controlled clinical trial. Eur J Clin Pharmacol. 2014 Jun;70(6):655-65. https://pubmed.ncbi.nlm.nih.gov/24643636/
- Gonzalez A, Mata S, Sanchez P, et al. Omega-3 fatty acids as adjunctive of antidepressant therapy and its effects on brain-derived neurotrophic factor in serum, monocytes and lymphocytes. Archivos Venezolanos de Farmacologia y Terapeutica 2011;30(4):72-8. https://www.researchgate.net/publication/288224363
- Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007 Oct 1;31(7):1393-6. https://pubmed.ncbi.nlm.nih.gov/17659823/
- Jahangard L, Sadeghi A, Ahmadpanah M, et al. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders - Results from a double-blind, randomized and placebo-controlled clinical trial. J Psychiatr Res. 2018 Dec;107:48-56. https://pubmed.ncbi.nlm.nih.gov/30317101/
- Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008 Mar;42(3):192-8. https://pubmed.ncbi.nlm.nih.gov/18247193/
- Jiang W, Whellan DJ, Adams KF, et al. Long-Chain Omega-3 Fatty Acid Supplements in Depressed Heart Failure Patients: Results of the OCEAN Trial. JACC Heart Fail. 2018 Oct;6(10):833-843. https://pubmed.ncbi.nlm.nih.gov/30098961/
- Kamath J. Omega 3 FA supplements as augmentation in the treatment of depression. ClinicalTrials.gov/show/NCT01803711 (first received 04 March 2013).
- Lespérance F, Frasure-Smith N, St-André E, et al. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry. 2011 Aug;72(8):1054-62. https://pubmed.ncbi.nlm.nih.gov/20584525/
- Lucas M, Asselin G, Mérette C, Poulin MJ, Dodin S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Am J Clin Nutr. 2009 Feb;89(2):641-51. https://pubmed.ncbi.nlm.nih.gov/19116322/
- Marangell LB, Martinez JM, Zboyan HA, et al. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003 May;160(5):996-8. https://pubmed.ncbi.nlm.nih.gov/12727707/
- Masoumi SZ, Kazemi F, Tavakolian S, et al. Effect of Citalopram in Combination with Omega-3 on Depression in Post-menopausal Women: A Triple Blind Randomized Controlled Trial. J Clin Diagn Res. 2016 Oct;10(10):QC01-QC05. https://pubmed.ncbi.nlm.nih.gov/27891399/
- Mazereeuw G, Herrmann N, Oh PI, et al. Omega-3 Fatty Acids, Depressive Symptoms, and Cognitive Performance in Patients With Coronary Artery Disease: Analyses From a Randomized, Double-Blind, Placebo-Controlled Trial. J Clin Psychopharmacol. 2016 Oct;36(5):436-44. https://pubmed.ncbi.nlm.nih.gov/27529771/
- Mischoulon D, Nierenberg AA, Schettler PJ, et al. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J Clin Psychiatry. 2015 Jan;76(1):54-61. https://pubmed.ncbi.nlm.nih.gov/25272149/
- Mischoulon D, Papakostas GI, Dording CM, et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J Clin Psychiatry. 2009 Dec;70(12):1636-44. https://pubmed.ncbi.nlm.nih.gov/19709502/
- Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002 Mar;159(3):477-9. https://pubmed.ncbi.nlm.nih.gov/11870016/
- Park Y, Park YS, Kim SH, et al. Supplementation of n-3 Polyunsaturated Fatty Acids for Major Depressive Disorder: A Randomized, Double-Blind, 12-Week, Placebo-Controlled Trial in Korea. Ann Nutr Metab. 2015;66(2-3):141-148. https://pubmed.ncbi.nlm.nih.gov/25824637/
- Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002 Oct;59(10):913-9. https://pubmed.ncbi.nlm.nih.gov/12365878/
- Rondanelli M, Giacosa A, Opizzi A, et al. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr. 2010 Feb;29(1):55-64. https://pubmed.ncbi.nlm.nih.gov/20595646/
- Shinto L, Marracci G, Mohr DC, et al. Omega-3 Fatty Acids for Depression in Multiple Sclerosis: A Randomized Pilot Study. PLoS One. 2016 Jan 22;11(1):e0147195. https://pubmed.ncbi.nlm.nih.gov/26799942/
- Silvers KM, Woolley CC, Hamilton FC, et al. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids. 2005 Mar;72(3):211-8. https://pubmed.ncbi.nlm.nih.gov/15664306/
- Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003 Aug;13(4):267-71. https://pubmed.ncbi.nlm.nih.gov/12888186/
- Qi L, Zhang Q, Zheng Z, et al. Treatment of Chinese Patients with Hypertriglyceridemia with a Pharmaceutical-Grade Preparation of Highly Purified Omega-3 Polyunsaturated Fatty Acid Ethyl Esters: Main Results of a Randomized, Double-Blind, Controlled Trial. Vasc Health Risk Manag. 2021 Sep 15;17:571-580. https://pubmed.ncbi.nlm.nih.gov/34552329/
- GABA P, Bhatt DL, Giugliano RP, et al. Comparative Reductions in Investigator-Reported and Adjudicated Ischemic Events in REDUCE-IT. J Am Coll Cardiol. 2021 Oct 12;78(15):1525-1537. https://pubmed.ncbi.nlm.nih.gov/34620410/
- Zhang HJ, Gao X, Guo XF, Li KL, Li S, Sinclair AJ, Li D. Effects of dietary eicosapentaenoic acid and docosahexaenoic acid supplementation on metabolic syndrome: A systematic review and meta-analysis of data from 33 randomized controlled trials. Clin Nutr. 2021 Jul;40(7):4538-4550. https://pubmed.ncbi.nlm.nih.gov/34229258/
- Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002 Aug;76(2):326-30. https://pubmed.ncbi.nlm.nih.gov/12145002/
- Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000 May;71(5):1085-94. https://pubmed.ncbi.nlm.nih.gov/10799369/
- Buckley R, Shewring B, Turner R, et al. Circulating triacylglycerol and apoE levels in response to EPA and docosahexaenoic acid supplementation in adult human subjects. Br J Nutr. 2004 Sep;92(3):477-83. https://pubmed.ncbi.nlm.nih.gov/15469651/
- Brinton EA, Ballantyne CM, Guyton JR, et al. Lipid Effects of Icosapent Ethyl in Women with Diabetes Mellitus and Persistent High Triglycerides on Statin Treatment: ANCHOR Trial Subanalysis. J Womens Health (Larchmt). 2018 Sep;27(9):1170-1176. https://pubmed.ncbi.nlm.nih.gov/29583081/
- Davidson MH, Maki KC, Kalkowski J, Schaefer EJ, et al. Effects of docosahexaenoic acid on serum lipoproteins in patients with combined hyperlipidemia: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. 1997 Jun;16(3):236-43. https://pubmed.ncbi.nlm.nih.gov/9176830/
- Grimsgaard S, Bonaa KH, Hansen JB, Nordøy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr. 1997 Sep;66(3):649-59. https://pubmed.ncbi.nlm.nih.gov/9280188/
- Geppert J, Kraft V, Demmelmair H, Koletzko B. Microalgal docosahexaenoic acid decreases plasma triacylglycerol in normolipidaemic vegetarians: a randomised trial. Br J Nutr. 2006 Apr;95(4):779-86. https://pubmed.ncbi.nlm.nih.gov/16571158/
- Golzari MH, Hosseini S, Koohdani F, et al. The Effect of Eicosapentaenoic Acid on the Serum Levels and Enzymatic Activity of Paraoxonase 1 in the Patients With Type 2 Diabetes Mellitus. Acta Med Iran. 2017 Aug;55(8):486-495. https://pubmed.ncbi.nlm.nih.gov/29034644/
- Kelley DS, Siegel D, Vemuri M, Mackey BE. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am J Clin Nutr. 2007 Aug;86(2):324-33. https://pubmed.ncbi.nlm.nih.gov/17684201/
- Mita T, Watada H, Ogihara T, et al. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007 Mar;191(1):162-7. https://pubmed.ncbi.nlm.nih.gov/16616147/
- Maki KC, Van Elswyk ME, McCarthy D, et al. Lipid responses to a dietary docosahexaenoic acid supplement in men and women with below average levels of high density lipoprotein cholesterol. J Am Coll Nutr. 2005 Jun;24(3):189-99. https://pubmed.ncbi.nlm.nih.gov/15930485/
- Mocking RJ, Assies J, Bot M, et al. Biological effects of add-on eicosapentaenoic acid supplementation in diabetes mellitus and co-morbid depression: a randomized controlled trial. PLoS One. 2012;7(11):e49431. https://pubmed.ncbi.nlm.nih.gov/23209576/
- Miller M, Ballantyne CM, Bays HE, et al. Effects of Icosapent Ethyl (Eicosapentaenoic Acid Ethyl Ester) on Atherogenic Lipid/Lipoprotein, Apolipoprotein, and Inflammatory Parameters in Patients With Elevated High-Sensitivity C-Reactive Protein (from the ANCHOR Study). Am J Cardiol. 2019 Sep 1;124(5):696-701. https://pubmed.ncbi.nlm.nih.gov/31277790/
- Martorell M, Capó X, Sureda A, et al. Effect of DHA on plasma fatty acid availability and oxidative stress during training season and football exercise. Food Funct. 2014 Aug;5(8):1920-31. https://pubmed.ncbi.nlm.nih.gov/24955731/
- Neff LM, Culiner J, Cunningham-Rundles S, et al. Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. J Nutr. 2011 Feb;141(2):207-13. https://pubmed.ncbi.nlm.nih.gov/21178084/
- Satoh N, Shimatsu A, Kotani K, et al. Highly purified eicosapentaenoic acid reduces cardio-ankle vascular index in association with decreased serum amyloid A-LDL in metabolic syndrome. Hypertens Res. 2009 Nov;32(11):1004-8. https://pubmed.ncbi.nlm.nih.gov/19763135/
- Satoh N, Shimatsu A, Kotani K, et al. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care. 2007 Jan;30(1):144-6. https://pubmed.ncbi.nlm.nih.gov/17192349/
- Sarbolouki S, Javanbakht MH, Derakhshanian H, et al. Eicosapentaenoic acid improves insulin sensitivity and blood sugar in overweight type 2 diabetes mellitus patients: a double-blind randomised clinical trial. Singapore Med J. 2013 Jul;54(7):387-90. https://pubmed.ncbi.nlm.nih.gov/23900468/
- Sawada T, Tsubata H, Hashimoto N, et al. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc Diabetol. 2016 Aug 26;15(1):121. https://pubmed.ncbi.nlm.nih.gov/27565734/
- Singhal A, Lanigan J, Storry C, et al. Docosahexaenoic acid supplementation, vascular function and risk factors for cardiovascular disease: a randomized controlled trial in young adults. J Am Heart Assoc. 2013 Jul 1;2(4):e000283. https://pubmed.ncbi.nlm.nih.gov/23817470/
- Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am J Clin Nutr. 2004 May;79(5):765-73. https://pubmed.ncbi.nlm.nih.gov/15113713/
- Sanders TA, Gleason K, Griffin B, Miller GJ. Influence of an algal triacylglycerol containing docosahexaenoic acid (22 : 6n-3) and docosapentaenoic acid (22 : 5n-6) on cardiovascular risk factors in healthy men and women. Br J Nutr. 2006 Mar;95(3):525-31. https://pubmed.ncbi.nlm.nih.gov/16512939/
- Tani S, Nagao K, Matsumoto M, Hirayama A. Highly purified eicosapentaenoic acid may increase low-density lipoprotein particle size by improving triglyceride metabolism in patients with hypertriglyceridemia. Circ J. 2013;77(9):2349-57. https://pubmed.ncbi.nlm.nih.gov/23811682/
- Theobald HE, Chowienczyk PJ, Whittall R, Humphries SE, Sanders TA. LDL cholesterol-raising effect of low-dose docosahexaenoic acid in middle-aged men and women. Am J Clin Nutr. 2004 Apr;79(4):558-63. https://pubmed.ncbi.nlm.nih.gov/15051597/
- Tani S, Nagao K, Kawauchi K, et al. The Ratio of Eicosapentaenoic Acid (EPA) to Arachidonic Acid may be a Residual Risk Marker in Stable Coronary Artery Disease Patients Receiving Treatment with Statin Following EPA Therapy. Am J Cardiovasc Drugs. 2017 Oct;17(5):409-420. https://pubmed.ncbi.nlm.nih.gov/28634822/
- Tomiyama H, Takazawa K, Osa S, et al. Do eicosapentaenoic acid supplements attenuate age-related increases in arterial stiffness in patients with dyslipidemia?: A preliminary study. Hypertens Res. 2005 Aug;28(8):651-5. https://pubmed.ncbi.nlm.nih.gov/16392769/
- Tsunoda F, Lamon-Fava S, Asztalos BF, et al. Effects of oral eicosapentaenoic acid versus docosahexaenoic acid on human peripheral blood mononuclear cell gene expression. Atherosclerosis. 2015 Aug;241(2):400-8. https://pubmed.ncbi.nlm.nih.gov/26074314/
- Véricel E, Colas R, Calzada C, et al. Moderate oral supplementation with docosahexaenoic acid improves platelet function and oxidative stress in type 2 diabetic patients. Thromb Haemost. 2015 Aug;114(2):289-96. https://pubmed.ncbi.nlm.nih.gov/25832443/
- Yamamoto T, Kajikawa Y, Otani S, et al. Protective effect of eicosapentaenoic acid on insulin resistance in hyperlipidemic patients and on the postoperative course of cardiac surgery patients: the possible involvement of adiponectin. Acta Med Okayama. 2014 Dec;68(6):349-61. https://pubmed.ncbi.nlm.nih.gov/25519029/
- Abbott KA, Burrows TL, Acharya S, et al. DHA-enriched fish oil reduces insulin resistance in overweight and obese adults. Prostaglandins Leukot Essent Fatty Acids. 2020 Aug;159:102154. https://pubmed.ncbi.nlm.nih.gov/32563863/
- Rao A, Briskey D, Nalley JO, Ganuza E. Omega-3 Eicosapentaenoic Acid (EPA) Rich Extract from the Microalga Nannochloropsis Decreases Cholesterol in Healthy Individuals: A Double-Blind, Randomized, Placebo-Controlled, Three-Month Supplementation Study. Nutrients. 2020 Jun 23;12(6):1869. https://pubmed.ncbi.nlm.nih.gov/32585854/
- Thota RN, Rosato JI, Burrows TL, et al. Docosahexaenoic Acid-Rich Fish Oil Supplementation Reduces Kinase Associated with Insulin Resistance in Overweight and Obese Midlife Adults. Nutrients. 2020 May 30;12(6):1612. https://pubmed.ncbi.nlm.nih.gov/32486256/
- Sedighiyan M, Abdollahi H, Karimi E, et al. Omega-3 polyunsaturated fatty acids supplementation improve clinical symptoms in patients with Covid-19: A randomised clinical trial. Int J Clin Pract. 2021 Dec;75(12):e14854. https://pubmed.ncbi.nlm.nih.gov/34516692/
- Calder PC: Omega-3 fatty acids and inflammatory processes. Nutrients. 2010 Mar;2(3):355-74. https://pubmed.ncbi.nlm.nih.gov/22254027
- Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE: DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr. 2009 Mar;139(3):495-501. https://pubmed.ncbi.nlm.nih.gov/19158225
- Picq M, Chen P, Perez M, Michaud M, Vericel E, Guichardant M, Lagarde M: DHA metabolism: targeting the brain and lipoxygenation. Mol Neurobiol. 2010 Aug;42(1):48-51. https://pubmed.ncbi.nlm.nih.gov/20422316
- Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N Jr: Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001 Aug;42(8):1257-65. https://pubmed.ncbi.nlm.nih.gov/11483627
- Pawlosky RJ, Hibbeln JR, Salem N Jr: Compartmental analyses of plasma n-3 essential fatty acids among male and female smokers and nonsmokers. J Lipid Res. 2007 Apr;48(4):935-43. Epub 2007 Jan 17. https://pubmed.ncbi.nlm.nih.gov/17234605
- Arterburn LM, Hall EB, Oken H: Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006 Jun;83(6 Suppl):1467S-1476S. https://pubmed.ncbi.nlm.nih.gov/16841856
- Braeckman RA, Stirtan WG, Soni PN: Pharmacokinetics of Eicosapentaenoic Acid in Plasma and Red Blood Cells After Multiple Oral Dosing With Icosapent Ethyl in Healthy Subjects. Clin Pharmacol Drug Dev. 2014 Mar;3(2):101-108. https://pubmed.ncbi.nlm.nih.gov/26097787
- Roberts KE, Adsett IT, Rickett K, et al. Systemic therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst Rev. 2022 Jan 10;1(1):CD013167. https://pubmed.ncbi.nlm.nih.gov/35005781/
- Ivanisevic M, Horvaticek M, Delmis K, Delmis J. Supplementation of EPA and DHA in pregnant women with type 1 diabetes mellitus. Ann Med. 2021 Dec;53(1):848-859. https://pubmed.ncbi.nlm.nih.gov/34210228/
- Kulkarni AV, Anand L, Vyas AK, et al. Omega-3 fatty acid lipid emulsions are safe and effective in reducing endotoxemia and sepsis in acute-on-chronic liver failure: An open-label randomized controlled trial. J Gastroenterol Hepatol. 2021 Jul;36(7):1953-1961. https://pubmed.ncbi.nlm.nih.gov/33450081/
- Gupta M, Liti B, Barrett C, et al. Prevention and Management of Hypertriglyceridemia-Induced Acute Pancreatitis during Pregnancy: A Systematic Review. Am J Med. 2022 Jan 23:S0002-9343(22)00039-0. https://pubmed.ncbi.nlm.nih.gov/35081380/
- Goldberg AS, Hegele RA. Severe hypertriglyceridemia in pregnancy. J Clin Endocrinol Metab. 2012 Aug;97(8):2589-96. https://pubmed.ncbi.nlm.nih.gov/22639290/
- Block RC, Shearer GC, Holub A, et al. Aspirin and omega-3 fatty acid status interact in the prevention of cardiovascular diseases in Framingham Heart Study. Prostaglandins Leukot Essent Fatty Acids. 2021 Jun;169:102283. https://pubmed.ncbi.nlm.nih.gov/33964664/
- Kalstad AA, Myhre PL, Laake K, et al; OMEMI Investigators. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction: A Randomized, Controlled Trial. Circulation. 2021 Feb 9;143(6):528-539. https://pubmed.ncbi.nlm.nih.gov/33191772/
- Verma S, Bhatt DL, Steg PG, et al; REDUCE-IT Investigators. Icosapent Ethyl Reduces Ischemic Events in Patients with a History of Previous Coronary Artery Bypass Grafting: REDUCE-IT CABG. Circulation. 2021 Dec 7;144(23):1845-1855. https://pubmed.ncbi.nlm.nih.gov/34710343/
- Budoff MJ, Muhlestein JB, Bhatt DL, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: a prospective, placebo-controlled randomized trial (EVAPORATE): interim results. Cardiovasc Res. 2021 Mar 21;117(4):1070-1077. https://pubmed.ncbi.nlm.nih.gov/32609331/
- Majithia A, Bhatt DL, Friedman AN, et al. Benefits of Icosapent Ethyl Across the Range of Kidney Function in Patients with Established Cardiovascular Disease or Diabetes: REDUCE-IT RENAL. Circulation. 2021 Nov 30;144(22):1750-1759. https://pubmed.ncbi.nlm.nih.gov/34706555/
- Liao J, Xiong Q, Yin Y, Ling Z, Chen S. The Effects of Fish Oil on Cardiovascular Diseases: Systematical Evaluation and Recent Advance. Front Cardiovasc Med. 2022 Jan 5;8:802306. http://pubmed.ncbi.nlm.nih.gov/35071366/
- Matthan NR, Jordan H, Chung M, et al. A systematic review and meta-analysis of the impact of omega-3 fatty acids on selected arrhythmia outcomes in animal models. Metabolism. 2005 Dec;54(12):1557-65. https://pubmed.ncbi.nlm.nih.gov/16311086/
- Cao H, Wang X, Huang H, et al. Omega-3 fatty acids in the prevention of atrial fibrillation recurrences after cardioversion: a meta-analysis of randomized controlled trials. Intern Med. 2012;51(18):2503-8. https://pubmed.ncbi.nlm.nih.gov/22989818/
- Costanzo S, di Niro V, Di Castelnuovo A, et al. Prevention of postoperative atrial fibrillation in open-heart surgery patients by preoperative supplementation of n-3 polyunsaturated fatty acids: an updated meta-analysis. J Thorac Cardiovasc Surg. 2013 Oct;146(4):906-11. https://pubmed.ncbi.nlm.nih.gov/23587470/
- Jia X, Gao F, Pickett JK, et al. Association Between Omega-3 Fatty Acid Treatment and Atrial Fibrillation in Cardiovascular Outcome Trials: A Systematic Review and Meta-Analysis. Cardiovasc Drugs Ther. 2021 Aug;35(4):793-800. https://pubmed.ncbi.nlm.nih.gov/34057665/
- Gencer B, Djousse L, Al-Ramady OT, Cook NR, Manson JE, Albert CM. Effect of Long-Term Marine ɷ-3 Fatty Acids Supplementation on the Risk of Atrial Fibrillation in Randomized Controlled Trials of Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Circulation. 2021 Dec 21;144(25):1981-1990. https://pubmed.ncbi.nlm.nih.gov/34612056/
- Khan SU, Lone AN, Khan MS, et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine. 2021 Jul 8;38:100997. https://pubmed.ncbi.nlm.nih.gov/34505026/
- Lombardi M, Chiabrando JG, Vescovo GM, et al. Impact of Different Doses of Omega-3 Fatty Acids on Cardiovascular Outcomes: a Pairwise and Network Meta-analysis. Curr Atheroscler Rep. 2020 Jul 16;22(9):45. https://pubmed.ncbi.nlm.nih.gov/32671519/
- Khawaja O, Gaziano JM, Djoussé L. A meta-analysis of omega-3 fatty acids and incidence of atrial fibrillation. J Am Coll Nutr. 2012 Feb;31(1):4-13. https://pubmed.ncbi.nlm.nih.gov/22661621/
- Li FR, Chen GC, Qin J, Wu X. Dietary Fish and Long-Chain n-3 Polyunsaturated Fatty Acids Intake and Risk of Atrial Fibrillation: A Meta-Analysis. Nutrients. 2017 Aug 29;9(9):955. https://pubmed.ncbi.nlm.nih.gov/28850090/
- León H, Shibata MC, Sivakumaran S, et al. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ. 2008 Dec 23;337:a2931. https://pubmed.ncbi.nlm.nih.gov/19106137/
- Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B. Omega 3 Fatty acids and cardiovascular outcomes: systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012 Nov;5(6):808-18. https://pubmed.ncbi.nlm.nih.gov/23110790/
- Khoueiry G, Abi Rafeh N, Sullivan E, et al. Do omega-3 polyunsaturated fatty acids reduce risk of sudden cardiac death and ventricular arrhythmias? A meta-analysis of randomized trials. Heart Lung. 2013 Jul-Aug;42(4):251-6. https://pubmed.ncbi.nlm.nih.gov/23714269/
- Liu T, Korantzopoulos P, Shehata M, et al. Prevention of atrial fibrillation with omega-3 fatty acids: a meta-analysis of randomised clinical trials. Heart. 2011 Jul;97(13):1034-40. https://pubmed.ncbi.nlm.nih.gov/21478384/
- Abdelhamid AS, Martin N, Bridges C, et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018 Nov 27;11(11):CD012345. https://pubmed.ncbi.nlm.nih.gov/30484282/
- Mariani J, Doval HC, Nul D, et al. N-3 polyunsaturated fatty acids to prevent atrial fibrillation: updated systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2013 Feb 19;2(1):e005033. https://pubmed.ncbi.nlm.nih.gov/23525440/
- Zhang B, Zhen Y, Tao A, et al. Polyunsaturated fatty acids for the prevention of atrial fibrillation after cardiac surgery: an updated meta-analysis of randomized controlled trials. J Cardiol. 2014 Jan;63(1):53-9. https://pubmed.ncbi.nlm.nih.gov/23911138/
- Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018 Jul 18;7(7):CD003177. https://pubmed.ncbi.nlm.nih.gov/30019766/
- Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2020 Feb 29;3(3):CD003177. https://pubmed.ncbi.nlm.nih.gov/32114706/
- Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018 Nov 30;11(11):CD003177. https://pubmed.ncbi.nlm.nih.gov/30521670/
- Armaganijan L, Lopes RD, Healey JS, et al. Do omega-3 fatty acids prevent atrial fibrillation after open heart surgery? A meta-analysis of randomized controlled trials. Clinics (Sao Paulo). 2011;66(11):1923-8. https://pubmed.ncbi.nlm.nih.gov/22086523/
- Guo XY, Yan XL, Chen YW, et al. Omega-3 fatty acids for postoperative atrial fibrillation: alone or in combination with antioxidant vitamins? Heart Lung Circ. 2014 Aug;23(8):743-50. https://pubmed.ncbi.nlm.nih.gov/24685324/
- He Z, Yang L, Tian J, et al. Efficacy and safety of omega-3 fatty acids for the prevention of atrial fibrillation: a meta-analysis. Can J Cardiol. 2013 Feb;29(2):196-203. https://pubmed.ncbi.nlm.nih.gov/22681963/
- Langlois PL, Hardy G, Manzanares W. Omega-3 polyunsaturated fatty acids in cardiac surgery patients: An updated systematic review and meta-analysis. Clin Nutr. 2017 Jun;36(3):737-746. https://pubmed.ncbi.nlm.nih.gov/27293143/
- Benedetto U, Angeloni E, Melina G, et al. n-3 Polyunsaturated fatty acids for the prevention of postoperative atrial fibrillation: a meta-analysis of randomized controlled trials. J Cardiovasc Med (Hagerstown). 2013 Feb;14(2):104-9. https://pubmed.ncbi.nlm.nih.gov/21826019/
- Wang H, Chen J, Zhao L. N-3 polyunsaturated fatty acids for prevention of postoperative atrial fibrillation: updated meta-analysis and systematic review. J Interv Card Electrophysiol. 2018 Mar;51(2):105-115. https://pubmed.ncbi.nlm.nih.gov/29380237/
- Kow CS, Doi SAR, Hasan SS. The coincidence of increased risk of atrial fibrillation in randomized control trials of omega-3 fatty acids: a meta-analysis. Expert Rev Clin Pharmacol. 2021 Jun;14(6):773-775. https://pubmed.ncbi.nlm.nih.gov/33798016/
- duplicate of 245
- Xiao F, Han W, Yue Q, Ke J, Jia B, Fu X. Perioperative omega-3 fatty acids for liver surgery: A meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021 Jul 9;100(27):e25743. https://pubmed.ncbi.nlm.nih.gov/34232163/
- Viecelli AK, Irish AB, Polkinghorne KR, et al. Omega-3 Polyunsaturated Fatty Acid Supplementation to Prevent Arteriovenous Fistula and Graft Failure: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am J Kidney Dis. 2018 Jul;72(1):50-61. https://pubmed.ncbi.nlm.nih.gov/29395485/
- Tam KW, Wu MY, Siddiqui FJ, Chan ES, Zhu Y, Jafar TH. Omega-3 fatty acids for dialysis vascular access outcomes in patients with chronic kidney disease. Cochrane Database Syst Rev. 2018 Nov 18;11(11):CD011353. https://pubmed.ncbi.nlm.nih.gov/30480758/
- Saglimbene VM, Wong G, van Zwieten A, et al. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: Systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2020 Feb;39(2):358-368. https://pubmed.ncbi.nlm.nih.gov/30905498/
- Alvarez Campano CG, Macleod MJ, Aucott L, Thies F. Marine-derived n-3 fatty acids therapy for stroke. Cochrane Database Syst Rev. 2019 Jun 26;6(6):CD012815. https://pubmed.ncbi.nlm.nih.gov/31242320/
- Villani AM, Crotty M, Cleland LG, James MJ, Fraser RJ, Cobiac L, Miller MD. Fish oil administration in older adults: is there potential for adverse events? A systematic review of the literature. BMC Geriatr. 2013 May 1;13:41. https://pubmed.ncbi.nlm.nih.gov/23634646/
- Alhabeeb H, Kord-Varkaneh H, Tan SC, et al. The influence of omega-3 supplementation on vitamin D levels in humans: a systematic review and dose-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2020 Dec 25:1-8. https://pubmed.ncbi.nlm.nih.gov/33356450/
- Li F, Pei L, Huang G, Ye H. Influence of omega-3 fatty acid and vitamin co-supplementation on metabolic status in gestational diabetes: A meta-analysis of randomized controlled studies. Eur J Obstet Gynecol Reprod Biol. 2020 Apr;247:191-197. https://pubmed.ncbi.nlm.nih.gov/32145487/
- Rajabi-Naeeni M, Dolatian M, Qorbani M, Vaezi AA. Effect of omega-3 and vitamin D co-supplementation on psychological distress in reproductive-aged women with pre-diabetes and hypovitaminosis D: A randomized controlled trial. Brain Behav. 2021 Nov;11(11):e2342. https://pubmed.ncbi.nlm.nih.gov/34473420/
- Rajabi-Naeeni M, Dolatian M, Qorbani M, Vaezi AA. The effect of omega-3 and vitamin D co-supplementation on glycemic control and lipid profiles in reproductive-aged women with pre-diabetes and hypovitaminosis D: a randomized controlled trial. Diabetol Metab Syndr. 2020 May 12;12:41. https://pubmed.ncbi.nlm.nih.gov/32435279/
- Jamilian M, Samimi M, Mirhosseini N, et al. The influences of vitamin D and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J Affect Disord. 2018 Oct 1;238:32-38. https://pubmed.ncbi.nlm.nih.gov/29859385/
- Jamilian M, Samimi M, Ebrahimi FA, et al. The effects of vitamin D and omega-3 fatty acid co-supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. J Clin Lipidol. 2017 Mar-Apr;11(2):459-468. https://pubmed.ncbi.nlm.nih.gov/28502503/
- Huang S, Fu J, Zhao R, Wang B, Zhang M, Li L, Shi C. The effect of combined supplementation with vitamin D and omega-3 fatty acids on blood glucose and blood lipid levels in patients with gestational diabetes. Ann Palliat Med. 2021 May;10(5):5652-5658. https://pubmed.ncbi.nlm.nih.gov/34107720/
- Haidari F, Abiri B, Iravani M, et al. Effects of Vitamin D and Omega-3 Fatty Acids Co-Supplementation on Inflammatory Factors and Tumor Marker CEA in Colorectal Cancer Patients Undergoing Chemotherapy: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutr Cancer. 2020;72(6):948-958. https://pubmed.ncbi.nlm.nih.gov/32441198/
- Haidari F, Abiri B, Iravani M, et al. Randomized Study Design to Test Effects of Vitamin D and Omega-3 Fatty Acid Supplementation as Adjuvant Therapy in Colorectal Cancer Patients. Methods Mol Biol. 2020;2138:337-350. https://pubmed.ncbi.nlm.nih.gov/32219761/
- duplicate of 138
- Mazahery H, Conlon CA, Beck KL, Mugridge O, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019 Mar;187:9-16. https://pubmed.ncbi.nlm.nih.gov/30744880/
- Infante M, Sears B, Rizzo AM, et al. Omega-3 PUFAs and vitamin D co-supplementation as a safe-effective therapeutic approach for core symptoms of autism spectrum disorder: case report and literature review. Nutr Neurosci. 2020 Oct;23(10):779-790. https://pubmed.ncbi.nlm.nih.gov/30545280/
- Djoussé L, Cook NR, Kim E, et al; VITAL Research Group. Supplementation With Vitamin D and Omega-3 Fatty Acids and Incidence of Heart Failure Hospitalization: VITAL-Heart Failure. Circulation. 2020 Mar 3;141(9):784-786. https://pubmed.ncbi.nlm.nih.gov/31709816/
- Rist PM, Buring JE, Cook NR, Manson JE, Rexrode KM. Effect of vitamin D and/or omega-3 fatty acid supplementation on stroke outcomes: A randomized trial. Eur J Neurol. 2021 Mar;28(3):809-815. https://pubmed.ncbi.nlm.nih.gov/33131164/
- Bassuk SS, Chandler PD, Buring JE, Manson JE; VITAL Research Group. The VITamin D and OmegA-3 TriaL (VITAL): Do Results Differ by Sex or Race/Ethnicity? Am J Lifestyle Med. 2020 Dec 24;15(4):372-391. https://pubmed.ncbi.nlm.nih.gov/34366734/
- Hahn J, Cook NR, Alexander EK, et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. 2022 Jan 26;376:e066452. https://pubmed.ncbi.nlm.nih.gov/35082139/
- MacFarlane LA, Cook NR, Kim E, Lee IM, Iversen MD, Gordon D, Buring JE, Katz JN, Manson JE, Costenbader KH. The Effects of Vitamin D and Marine Omega-3 Fatty Acid Supplementation on Chronic Knee Pain in Older US Adults: Results From a Randomized Trial. Arthritis Rheumatol. 2020 Nov;72(11):1836-1844. https://pubmed.ncbi.nlm.nih.gov/32583982/
- Chandler PD, Chen WY, Ajala ON, et al; VITAL Research Group. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw Open. 2020 Nov 2;3(11):e2025850. https://pubmed.ncbi.nlm.nih.gov/33206192/
- LeBoff MS, Murata EM, Cook NR, Cawthon P, Chou SH, Kotler G, Bubes V, Buring JE, Manson JE. VITamin D and OmegA-3 TriaL (VITAL): Effects of Vitamin D Supplements on Risk of Falls in the US Population. J Clin Endocrinol Metab. 2020 Sep 1;105(9):2929–38. https://pubmed.ncbi.nlm.nih.gov/32492153/
- Rist PM, Buring JE, Cook NR, Manson JE, Kurth T. Effect of Vitamin D and/or Marine n-3 Fatty Acid Supplementation on Changes in Migraine Frequency and Severity. Am J Med. 2021 Jun;134(6):756-762.e5. https://pubmed.ncbi.nlm.nih.gov/33444588/
- Sujeta A, Capkauskiene S, Vizbaraite D, et al. Low-Dose Omega-3 Fatty Acid and Vitamin D for Anthropometric, Biochemical Blood Indices and Respiratory Function. Does it work? Int J Vitam Nutr Res. 2020 Jan;90(1-2):67-83. https://pubmed.ncbi.nlm.nih.gov/30932776/
- Lee J, Lee SI. Efficacy of Omega-3 and Korean Red Ginseng in Children with Subthreshold ADHD: A Double-Blind, Randomized, Placebo-Controlled Trial. J Atten Disord. 2021 Dec;25(14):1977-1987. https://pubmed.ncbi.nlm.nih.gov/32847461/
- Carmichael OT, Pillai S, Shankapal P, McLellan A, Kay DG, Gold BT, Keller JN. A Combination of Essential Fatty Acids, Panax ginseng Extract, and Green Tea Catechins Modifies Brain fMRI Signals in Healthy Older Adults. J Nutr Health Aging. 2018;22(7):837-846. https://pubmed.ncbi.nlm.nih.gov/30080229/
- Tański W, Świątoniowska-Lonc N, Tabin M, Jankowska-Polańska B. The Relationship between Fatty Acids and the Development, Course and Treatment of Rheumatoid Arthritis. Nutrients. 2022 Feb 28;14(5):1030. https://pubmed.ncbi.nlm.nih.gov/35268002/
- Chatterjee D, Chatterjee A, Kalra D, et al. Role of adjunct use of omega 3 fatty acids in periodontal therapy of periodontitis. A systematic review and meta-analysis. J Oral Biol Craniofac Res. 2022 Jan-Feb;12(1):55-62. https://pubmed.ncbi.nlm.nih.gov/34760614/
- Elwakeel NM, Hazaa HH. Effect of omega 3 fatty acids plus low-dose aspirin on both clinical and biochemical profile of patients with chronic periodontitis and type 2 diabetes: a randomized double blind placebo-controlled study. J Periodontal Res. 2015;50:721–729. https://pubmed.ncbi.nlm.nih.gov/25604769/
- El-Sharkawy Hesham. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 fatty acids and low dose aspirin. J Periodontol. 2010;81:1635–1643. https://pubmed.ncbi.nlm.nih.gov/20572767/
- Elkhouli AM. The efficacy of host response modulation therapy as an adjunctive treatment of chronic periodontitis: a randomized, double –blind, placebo-controlled study. J Periodontal Res2011; 46:261-268. https://pubmed.ncbi.nlm.nih.gov/21261621/
- Castro dos Santos Nidia C. Omega -3 PUFA and aspirin as adjuncts to periodontal debridement in patients with periodontitis and type 2 diabetes mellitus. Randomized clinical trial. J Periodontol. 2020;91:1318–1327. https://pubmed.ncbi.nlm.nih.gov/32103495/
- Proudman SM, James MJ, Spargo LD, et al. Fish oil in recent onset rheumatoid arthritis: a randomised, double-blind controlled trial within algorithm-based drug use. Ann Rheum Dis. 2015 Jan;74(1):89-95. http://pubmed.ncbi.nlm.nih.gov/24081439/
- Susai SR, Sabherwal S, Mongan D, et al. Omega-3 fatty acid in ultra-high-risk psychosis: A systematic review based on functional outcome. Early Interv Psychiatry. 2022 Jan;16(1):3-16. https://pubmed.ncbi.nlm.nih.gov/33652502/
- Susai SR, Mongan D, Healy C, et al. The association of plasma inflammatory markers with omega-3 fatty acids and their mediating role in psychotic symptoms and functioning: An analysis of the NEURAPRO clinical trial. Brain Behav Immun. 2022 Jan;99:147-156. https://pubmed.ncbi.nlm.nih.gov/34624483/
- Xu X, Shao G, Zhang X, et al. The efficacy of nutritional supplements for the adjunctive treatment of schizophrenia in adults: A systematic review and network meta-analysis. Psychiatry Res. 2022 Mar 7;311:114500. https://pubmed.ncbi.nlm.nih.gov/35287043/
- Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019 Nov 1;2019(11):CD012236. https://pubmed.ncbi.nlm.nih.gov/31689359/
- Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: Possible stage-specific effects. Ann Clin Psychiatry. 2015 Nov;27(4):289-96. https://pubmed.ncbi.nlm.nih.gov/26554370/
- Jamilian H, Solhi H, Jamilian M. Randomized, placebo-controlled clinical trial of omega-3 as supplemental treatment in schizophrenia. Glob J Health Sci. 2014 Sep 18;6(7 Spec No):103-8. https://pubmed.ncbi.nlm.nih.gov/25363186/
- Peet M, Horrobin DF; E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002 Jan-Feb;36(1):7-18. https://pubmed.ncbi.nlm.nih.gov/11755456/
- Pawełczyk T, Grancow-Grabka M, Kotlicka-Antczak M, et al. A randomized controlled study of the efficacy of six-month supplementation with concentrated fish oil rich in omega-3 polyunsaturated fatty acids in first episode schizophrenia. J Psychiatr Res. 2016 Feb;73:34-44. https://pubmed.ncbi.nlm.nih.gov/26679763/
- Cuéllar-Barboza AB, Sánchez-Ruiz JA, Corral PM. Use of Omega-3 Polyunsaturated Fatty Acids as Augmentation Therapy in Treatment-Resistant Schizophrenia. Prim Care Companion CNS Disord. 2017 May 4;19(3). https://pubmed.ncbi.nlm.nih.gov/28472557/
- Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res. 2001 Apr 30;49(3):243-51. https://pubmed.ncbi.nlm.nih.gov/11356585/
- Su KP, Shen WW, Huang SY. Omega-3 fatty acids as a psychotherapeutic agent for a pregnant schizophrenic patient. Eur Neuropsychopharmacol. 2001 Aug;11(4):295-9. https://pubmed.ncbi.nlm.nih.gov/11532384/
- Sivrioglu EY, Kirli S, Sipahioglu D, et al. The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: an open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007 Oct 1;31(7):1493-9. https://pubmed.ncbi.nlm.nih.gov/17688987/
- Lyall AE, Nägele FL, Pasternak O, et al. A 16-week randomized placebo-controlled trial investigating the effects of omega-3 polyunsaturated fatty acid treatment on white matter microstructure in recent-onset psychosis patients concurrently treated with risperidone. Psychiatry Res Neuroimaging. 2021 Jan 30;307:111219. https://pubmed.ncbi.nlm.nih.gov/33221631/
- Reddy R, Fleet-Michaliszyn S, Condray R, et al. Reduction in perseverative errors with adjunctive ethyl-eicosapentaenoic acid in patients with schizophrenia: Preliminary study. Prostaglandins Leukot Essent Fatty Acids. 2011 Mar-Apr;84(3-4):79-83. https://pubmed.ncbi.nlm.nih.gov/21211955/
- Tang W, Wang Y, Xu F, et al. Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain Behav Immun. 2020 Aug;88:529-534. https://pubmed.ncbi.nlm.nih.gov/32304881/
- Bellino S, Bozzatello P, Badino C, Mantelli E, Rocca P. Efficacy of Polyunsaturated Fatty Acids (PUFAs) on Impulsive Behaviours and Aggressiveness in Psychiatric Disorders. Int J Mol Sci. 2021 Jan 9;22(2):620. https://pubmed.ncbi.nlm.nih.gov/33435512/
- Robinson DG, Gallego JA, John M, et al. A potential role for adjunctive omega-3 polyunsaturated fatty acids for depression and anxiety symptoms in recent onset psychosis: Results from a 16 week randomized placebo-controlled trial for participants concurrently treated with risperidone. Schizophr Res. 2019 Feb;204:295-303. https://pubmed.ncbi.nlm.nih.gov/30241990/
- Peet M. Eicosapentaenoic acid in the treatment of schizophrenia and depression: rationale and preliminary double-blind clinical trial results. Prostaglandins Leukot Essent Fatty Acids. 2003 Dec;69(6):477-85. https://pubmed.ncbi.nlm.nih.gov/14623502/
- Freeman MP, McInerney K, Sosinsky AZ, et al. Omega-3 fatty acids for atypical antipsychotic-associated hypertriglyceridemia. Ann Clin Psychiatry. 2015 Aug;27(3):197-202. https://pubmed.ncbi.nlm.nih.gov/26247219/
- Pawełczyk T, Grancow-Grabka M, Żurner N, Pawełczyk A. Omega-3 fatty acids reduce cardiometabolic risk in first-episode schizophrenia patients treated with antipsychotics: Findings from the OFFER randomized controlled study. Schizophr Res. 2021 Apr;230:61-68. https://pubmed.ncbi.nlm.nih.gov/33684737/
- Xu F, Fan W, Wang W, et al. Effects of omega-3 fatty acids on metabolic syndrome in patients with schizophrenia: a 12-week randomized placebo-controlled trial. Psychopharmacology (Berl). 2019 Apr;236(4):1273-1279. https://pubmed.ncbi.nlm.nih.gov/30519766/
- Behdani F, Roudbaraki SN, Saberi-Karimian M, et al. Assessment of the efficacy of omega-3 fatty acids on metabolic and inflammatory parameters in patients with schizophrenia taking clozapine and sodium valproate. Psychiatry Res. 2018 Mar;261:243-247. https://pubmed.ncbi.nlm.nih.gov/29329042/
- Caniato RN, Alvarenga ME, Garcia-Alcaraz MA. Effect of omega-3 fatty acids on the lipid profile of patients taking clozapine. Aust N Z J Psychiatry. 2006 Aug;40(8):691-7. https://pubmed.ncbi.nlm.nih.gov/16866765/
- Fetter JC, Brunette M, Green AI. N-3 fatty acids for hypertriglyceridemia in patients taking second-generation antipsychotics. Clin Schizophr Relat Psychoses. 2013 Summer;7(2):73-77A. https://pubmed.ncbi.nlm.nih.gov/23367502/
- Tse L, Procyshyn RM, Fredrikson DH, et al. Pharmacological treatment of antipsychotic-induced dyslipidemia and hypertension. Int Clin Psychopharmacol. 2014 May;29(3):125-37. https://pubmed.ncbi.nlm.nih.gov/24169026/
- Pisano S, Gritti A, Catone G, Pascotto A. Antipsychotic-induced dyslipidemia treated with omega 3 fatty acid supplement in an 11-year-old psychotic child: a 1-year follow-up. J Child Adolesc Psychopharmacol. 2013 Mar;23(2):139-41. https://pubmed.ncbi.nlm.nih.gov/23480323/
- Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry. 2001 Dec;158(12):2071-4. https://pubmed.ncbi.nlm.nih.gov/11729030/
- Emsley R, Niehaus DJ, Koen L, et al. The effects of eicosapentaenoic acid in tardive dyskinesia: a randomized, placebo-controlled trial. Schizophr Res. 2006, May;84(1):112-20. https://pubmed.ncbi.nlm.nih.gov/16632329/
- Emsley R, Niehaus DJ, Oosthuizen PP, et al. Safety of the omega-3 fatty acid, eicosapentaenoic acid (EPA) in psychiatric patients: results from a randomized, placebo-controlled trial. Psychiatry Res. 2008 Dec 15;161(3):284-91. https://pubmed.ncbi.nlm.nih.gov/18962989/
- Song C, Shieh CH, et al. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer's disease: Acting separately or synergistically? Prog Lipid Res. 2016 Apr;62:41-54. http://pubmed.ncbi.nlm.nih.gov/26763196/
- Krawczyk K, Rybakowski J. Potencjalizacja leków przeciwdepresyjnych kwasami tłuszczowymi omega-3 w depresji lekoopornej [Augmentation of antidepressants with unsaturated fatty acids omega-3 in drug-resistant depression]. Psychiatr Pol. 2012 Jul-Aug;46(4):585-98. https://pubmed.ncbi.nlm.nih.gov/23214161/
- Jazayeri S, Keshavarz SA, Tehrani-Doost M, et al. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010 Jun 30;178(1):112-5. https://pubmed.ncbi.nlm.nih.gov/20466437/
- Hoffmire CA, Block RC, Thevenet-Morrison K, et al. Associations between omega-3 poly-unsaturated fatty acids from fish consumption and severity of depressive symptoms: an analysis of the 2005-2008 National Health and Nutrition Examination Survey. Prostaglandins Leukot Essent Fatty Acids. 2012 Apr;86(4-5):155-60. https://pubmed.ncbi.nlm.nih.gov/22472486/
- Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Hibbeln JR, Evans MK, Zonderman AB. ω-3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. J Nutr. 2013 Nov;143(11):1743-52. https://pubmed.ncbi.nlm.nih.gov/24005610/
- Chrysohoou C, Tsitsinakis G, Siassos G, et al. Fish Consumption Moderates Depressive Symptomatology in Elderly Men and Women from the IKARIA Study. Cardiol Res Pract. 2010 Dec 15;2011:219578. https://pubmed.ncbi.nlm.nih.gov/21197433/
- Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006 Oct;63(10):1402-8. https://pubmed.ncbi.nlm.nih.gov/17030655/
- Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010 Nov 3;304(17):1903-11. https://pubmed.ncbi.nlm.nih.gov/21045096/
- Irving GF, Freund-Levi Y, Eriksdotter-Jönhagen M, et al. Omega-3 fatty acid supplementation effects on weight and appetite in patients with Alzheimer's disease: the omega-3 Alzheimer's disease study. J Am Geriatr Soc. 2009 Jan;57(1):11-7. https://pubmed.ncbi.nlm.nih.gov/19054188/
- Vedin I, Cederholm T, Freund-Levi Y, et al. Effects of DHA-rich n-3 fatty acid supplementation on gene expression in blood mononuclear leukocytes: the OmegAD study. PLoS One. 2012;7(4):e35425. https://pubmed.ncbi.nlm.nih.gov/22545106/
- Vedin I, Cederholm T, Freund Levi Y, et al. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am J Clin Nutr. 2008 Jun;87(6):1616-22. https://pubmed.ncbi.nlm.nih.gov/18541548/
- Vedin I, Cederholm T, Freund-Levi Y, et al. Reduced prostaglandin F2 alpha release from blood mononuclear leukocytes after oral supplementation of omega3 fatty acids: the OmegAD study. J Lipid Res. 2010 May;51(5):1179-85. https://pubmed.ncbi.nlm.nih.gov/19965584/
- Freund Levi Y, Vedin I, Cederholm T, et al. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer's disease: the OmegAD study. J Intern Med. 2014 Apr;275(4):428-36. https://pubmed.ncbi.nlm.nih.gov/24410954/
- Lin PY, Cheng C, Satyanarayanan SK, et al. Omega-3 fatty acids and blood-based biomarkers in Alzheimer's disease and mild cognitive impairment: A randomized placebo-controlled trial. Brain Behav Immun. 2022 Jan;99:289-298. https://pubmed.ncbi.nlm.nih.gov/34755655/
- Lombardi M, Carbone S, Del Buono MG, et al. Omega-3 fatty acids supplementation and risk of atrial fibrillation: an updated meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. 2021 Jul 23;7(4):e69-e70. https://pubmed.ncbi.nlm.nih.gov/33910233/
- Fazelian S, Moradi F, Agah S, et al. Effect of omega-3 fatty acids supplementation on cardio-metabolic and oxidative stress parameters in patients with chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2021 May 1;22(1):160. https://pubmed.ncbi.nlm.nih.gov/33933009/
- Chiva-Blanch G, Bratseth V, Laake K, Arnesen H, et al. One year of omega 3 polyunsaturated fatty acid supplementation does not reduce circulating prothrombotic microvesicles in elderly subjects after suffering a myocardial infarction. Clin Nutr. 2021 Dec;40(12):5674-5677. https://pubmed.ncbi.nlm.nih.gov/34742136/
- Ma MY, Li KL, Zheng H, Dou YL, et al. Omega-3 index and type 2 diabetes: Systematic review and meta-analysis. Prostaglandins Leukot Essent Fatty Acids. 2021 Nov;174:102361. https://pubmed.ncbi.nlm.nih.gov/34740031/
- Kim Y, Oh YK, Lee J, Kim E. Could nutrient supplements provide additional glycemic control in diabetes management? A systematic review and meta-analysis of randomized controlled trials of as an add-on nutritional supplementation therapy. Arch Pharm Res. 2022 Mar;45(3):185-204. https://pubmed.ncbi.nlm.nih.gov/35304727/
- Takeshita Y, Teramura C, Kamoshita K, et al. Effects of eicosapentaenoic acid on serum levels of selenoprotein P and organ-specific insulin sensitivity in humans with dyslipidemia and type 2 diabetes. J Diabetes Investig. 2022 Mar;13(3):532-542. https://pubmed.ncbi.nlm.nih.gov/34670012/
- Shen S, Gong C, Jin K, et al. Omega-3 Fatty Acid Supplementation and Coronary Heart Disease Risks: A Meta-Analysis of Randomized Controlled Clinical Trials. Front Nutr. 2022 Feb 3;9:809311. https://pubmed.ncbi.nlm.nih.gov/35187035/
- Mozaffarian D, Maki KC, Bays HE, et al; TRILOGY (Study of CaPre in Lowering Very High Triglycerides) investigators. Effectiveness of a Novel ω-3 Krill Oil Agent in Patients with Severe Hypertriglyceridemia: A Randomized Clinical Trial. JAMA Netw Open. 2022 Jan 4;5(1):e2141898. https://pubmed.ncbi.nlm.nih.gov/34989797/
- Pourmasoumi M, Vosoughi N, Derakhshandeh-Rishehri SM, et al. Association of Omega-3 Fatty Acid and Epileptic Seizure in Epileptic Patients: A Systematic Review. Int J Prev Med. 2018 Apr 5;9:36. https://pubmed.ncbi.nlm.nih.gov/29721237/
- Bromfield E, Dworetzky B, Hurwitz S, et al. A randomized trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy Behav. 2008 Jan;12(1):187-90. https://pubmed.ncbi.nlm.nih.gov/18086463/
- DeGiorgio CM, Miller PR, Harper R, et al. Fish oil (n-3 fatty acids) in drug resistant epilepsy: a randomised placebo-controlled crossover study. J Neurol Neurosurg Psychiatry. 2015 Jan;86(1):65-70. https://pubmed.ncbi.nlm.nih.gov/25201887/
- Reda DM, Abd-El-Fatah NK, Omar Tel-S, Darwish OA. Fish Oil Intake and Seizure Control in Children with Medically Resistant Epilepsy. N Am J Med Sci. 2015 Jul;7(7):317-21. https://pubmed.ncbi.nlm.nih.gov/26258079/
- Dahlin M, Hjelte L, Nilsson S, Amark P. Plasma phospholipid fatty acids are influenced by a ketogenic diet enriched with n-3 fatty acids in children with epilepsy. Epilepsy Res. 2007 Feb;73(2):199-207. https://pubmed.ncbi.nlm.nih.gov/17150333/
- DeGiorgio CM, Miller P, Meymandi S, Gornbein JA. n-3 fatty acids (fish oil) for epilepsy, cardiac risk factors, and risk of SUDEP: clues from a pilot, double-blind, exploratory study. Epilepsy Behav. 2008 Nov;13(4):681-4. https://pubmed.ncbi.nlm.nih.gov/18721899/
- Yuen AW, Flugel D, Poepel A, Bell GS, Peacock JL, Sander JW. Non-randomized open trial of eicosapentaenoic acid (EPA), an omega-3 fatty acid, in ten people with chronic epilepsy. Epilepsy Behav. 2012 Mar;23(3):370-2. https://pubmed.ncbi.nlm.nih.gov/22342198/
- Yuen AW, Sander JW, Fluegel D, Patsalos PN, Bell GS, Johnson T, Koepp MJ. Omega-3 fatty acid supplementation in patients with chronic epilepsy: a randomized trial. Epilepsy Behav. 2005 Sep;7(2):253-8. https://pubmed.ncbi.nlm.nih.gov/16006194/
- Schlanger S, Shinitzky M, Yam D. Diet enriched with omega-3 fatty acids alleviates convulsion symptoms in epilepsy patients. Epilepsia. 2002 Jan;43(1):103-4. https://pubmed.ncbi.nlm.nih.gov/11879394/
- Puri BK, Koepp MJ, Holmes J, et al. A 31-phosphorus neurospectroscopy study of omega-3 long-chain polyunsaturated fatty acid intervention with eicosapentaenoic acid and docosahexaenoic acid in patients with chronic refractory epilepsy. Prostaglandins Leukot Essent Fatty Acids. 2007 Aug;77(2):105-7. https://pubmed.ncbi.nlm.nih.gov/17761409/
- Omrani S, Taheri M, Omrani MD, et al. The effect of omega-3 fatty acids on clinical and paraclinical features of intractable epileptic patients: a triple blind randomized clinical trial. Clin Transl Med. 2019 Jan 16;8(1):3. https://pubmed.ncbi.nlm.nih.gov/30649643/
- Ghafouri-Fard S, Hashemi M, Rafigh M, et al. Altered IFN-γ Levels after Treatment of Epileptic Patients with Omega-3 Fatty Acids. J Mol Neurosci. 2021 Nov;71(11):2364-2367. https://pubmed.ncbi.nlm.nih.gov/33580472/
- Delpino FM, Figueiredo LM. Effects of omega-3 supplementation on lean body mass in cancer patients: a systematic review and meta-analysis. Eur J Clin Nutr. 2022 Feb 16. https://pubmed.ncbi.nlm.nih.gov/35173292/
- de Castro GS, Andrade MF, Pinto FCS, et al. Omega-3 Fatty Acid Supplementation and Its Impact on Systemic Inflammation and Body Weight in Patients With Cancer Cachexia-A Systematic Review and Meta-Analysis. Front Nutr. 2022 Jan 31;8:797513. https://pubmed.ncbi.nlm.nih.gov/35174197/
- Carvalho TC, Cruz BC, Viana MS, et al. Effect of Nutritional Supplementation Enriched with Eicosapentaenoic Acid on Inflammatory Profile of Patients With Oral Cavity Cancer in Antineoplastic Pretreatment: A Controlled and Randomized Clinical Trial. Nutr Cancer. 2017 Apr;69(3):428-435. https://pubmed.ncbi.nlm.nih.gov/28128983/
- Faber J, Uitdehaag MJ, Spaander M, et al. Improved body weight and performance status and reduced serum PGE2 levels after nutritional intervention with a specific medical food in newly diagnosed patients with esophageal cancer or adenocarcinoma of the gastro-esophageal junction. J Cachexia Sarcopenia Muscle. 2015 Mar;6(1):32-44. https://pubmed.ncbi.nlm.nih.gov/26136410/
- Liu Y, Jia Z, Dong L, Wang R, Qiu G. A randomized pilot study of atractylenolide I on gastric cancer cachexia patients. Evid Based Complement Alternat Med. 2008 Sep;5(3):337-44. https://pubmed.ncbi.nlm.nih.gov/18830451/
- Persson C, Glimelius B, Rönnelid J, Nygren P. Impact of fish oil and melatonin on cachexia in patients with advanced gastrointestinal cancer: a randomized pilot study. Nutrition. 2005 Feb;21(2):170-8. https://pubmed.ncbi.nlm.nih.gov/15723745/
- duplicate of 151
- Yeh KY, Wang HM, Chang JW, et al. Omega-3 fatty acid-, micronutrient-, and probiotic-enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 Jul;116(1):41-8. https://pubmed.ncbi.nlm.nih.gov/23562359/
- Ueno M, Sugimori K, Taguri M, et al. Randomized Phase II Study of Gemcitabine Monotherapy vs. Gemcitabine with an EPA-Enriched Oral Supplement in Advanced Pancreatic Cancer. Nutr Cancer. 2022;74(1):122-130. https://pubmed.ncbi.nlm.nih.gov/33438442/
- Tan SE, Abdul Satar NF, Majid HA. Effects of Immunonutrition in Head and Neck Cancer Patients Undergoing Cancer Treatment - A Systematic Review. Front Nutr. 2022 Feb 25;9:821924. https://pubmed.ncbi.nlm.nih.gov/35360685/
- Sim E, Kim JM, Lee SM, et al. The Effect of Omega-3 Enriched Oral Nutrition Supplement on Nutritional Indices and Quality of Life in Gastrointestinal Cancer Patients: A Randomized Clinical Trial. Asian Pac J Cancer Prev. 2022 Feb 1;23(2):485-494. https://pubmed.ncbi.nlm.nih.gov/35225460/
- Dechaphunkul T, Arundon T, Raungkhajon P, et al. Benefits of immunonutrition in patients with head and neck cancer receiving chemoradiation: A phase II randomized, double-blind study. Clin Nutr. 2022 Feb;41(2):433-440. https://pubmed.ncbi.nlm.nih.gov/35007812/
- Watson H, Stackhouse C. Omega-3 fatty acid supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2020 Apr 10;4(4):CD002201. https://pubmed.ncbi.nlm.nih.gov/32275788/
- Hanssens L, Thiébaut I, Lefèvre N, et al. The clinical benefits of long-term supplementation with omega-3 fatty acids in cystic fibrosis patients - A pilot study. Prostaglandins Leukot Essent Fatty Acids. 2016 May;108:45-50. https://pubmed.ncbi.nlm.nih.gov/27154364/
- Henderson WR Jr, Astley SJ, McCready MM, et al. Oral absorption of omega-3 fatty acids in patients with cystic fibrosis who have pancreatic insufficiency and in healthy control subjects. J Pediatr. 1994 Mar;124(3):400-8. https://pubmed.ncbi.nlm.nih.gov/8120709/
- Keen C, Olin AC, Eriksson S, et al. Supplementation with fatty acids influences the airway nitric oxide and inflammatory markers in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010 May;50(5):537-44. https://pubmed.ncbi.nlm.nih.gov/20639712/
- Lawrence R, Sorrell T. Eicosapentaenoic acid in cystic fibrosis: evidence of a pathogenetic role for leukotriene B4. Lancet. 1993 Aug 21;342(8869):465-9. https://pubmed.ncbi.nlm.nih.gov/8102430/
- Panchaud A, Sauty A, Kernen Y, et al. Biological effects of a dietary omega-3 polyunsaturated fatty acids supplementation in cystic fibrosis patients: a randomized, crossover placebo-controlled trial. Clin Nutr. 2006 Jun;25(3):418-27. https://pubmed.ncbi.nlm.nih.gov/16325968/
- López-Neyra A, Suárez L, Muñoz M, et al. Long-term docosahexaenoic acid (DHA) supplementation in cystic fibrosis patients: a randomized, multi-center, double-blind, placebo-controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2020 Nov;162:102186. https://pubmed.ncbi.nlm.nih.gov/33038833/
- Olveira G, Olveira C, Acosta E, et al. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Arch Bronconeumol. 2010 Feb;46(2):70-7. https://pubmed.ncbi.nlm.nih.gov/20045240/
- Sedighi I, Taheri-Moghadam G, Emad-Momtaz H, et al. Protective Effects of Omega-3 Fatty Acids Supplementation Against Renal Parenchymal Scarring in Children with Acute Pyelonephritis: Results of a Pilot Clinical Trial. Curr Pediatr Rev. 2022;18(1):72-81. https://pubmed.ncbi.nlm.nih.gov/34503428/
- duplicate of 217
- duplicate of 305
- Qiao Y, Mei Y, Han H, Liu F, Yang XM, Shao Y, Xie B, Long B. Effects of Omega-3 in the treatment of violent schizophrenia patients. Schizophr Res. 2018 May;195:283-285. https://pubmed.ncbi.nlm.nih.gov/28830741/
- Amirkhani Z, Alavi M, Kalani M, et al. Immunomodulatory Effects of Omega-3 Fatty Acids in Patients with Differentiated Thyroid Cancer Before or After Radioiodine Ablation. Iran J Immunol. 2022 Mar;19(1):7. https://pubmed.ncbi.nlm.nih.gov/35293348/
- Mengelberg A, Leathem J, Podd J, Hill S, Conlon C. The effects of docosahexaenoic acid supplementation on cognition and well-being in mild cognitive impairment: A 12-month randomised controlled trial. Int J Geriatr Psychiatry. 2022 May;37(5). https://pubmed.ncbi.nlm.nih.gov/35373862/
- Pan L, Zhou Y, Yin H, Hui H, Guo Y, Xie X. Omega-3 Polyunsaturated Fatty Acids Can Reduce C-Reactive Protein in Patients with Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr Cancer. 2022;74(3):840-851. https://pubmed.ncbi.nlm.nih.gov/34060403/
- Kishi T, Sakuma K, Okuya M, et al. Omega-3 fatty acids for treating residual depressive symptoms in adult patients with bipolar disorder: A systematic review and meta-analysis of double-blind randomized, placebo-controlled trials. Bipolar Disord. 2021 Nov;23(7):730-731. https://pubmed.ncbi.nlm.nih.gov/34228881/
- McPhilemy G, Byrne F, Waldron M, et al. A 52-week prophylactic randomised control trial of omega-3 polyunsaturated fatty acids in bipolar disorder. Bipolar Disord. 2021 Nov;23(7):697-706. https://pubmed.ncbi.nlm.nih.gov/33340432/
- Saunders EFH, Mukherjee D, Myers T, et al. Adjunctive dietary intervention for bipolar disorder: a randomized, controlled, parallel-group, modified double-blinded trial of a high n-3 plus low n-6 diet. Bipolar Disord. 2022 Mar;24(2):171-184. https://pubmed.ncbi.nlm.nih.gov/34218509/
- Myhre PL, Kalstad AA, Tveit SH, et al. Changes in eicosapentaenoic acid and docosahexaenoic acid and risk of cardiovascular events and atrial fibrillation: A secondary analysis of the OMEMI trial. J Intern Med. 2022 May;291(5):637-647. https://pubmed.ncbi.nlm.nih.gov/34982486/
- Hearon CM Jr, Dias KA, MacNamara JP, et al. 1 Year HIIT and Omega-3 Fatty Acids to Improve Cardiometabolic Risk in Stage-A Heart Failure. JACC Heart Fail. 2022 Apr;10(4):238-249. https://pubmed.ncbi.nlm.nih.gov/35361442/
- Markozannes G, Ntzani EE, Tsapas A, et al. Dose-related meta-analysis for Omega-3 fatty acids supplementation on major adverse cardiovascular events. Clin Nutr. 2022 Apr;41(4):923-930. https://pubmed.ncbi.nlm.nih.gov/35290840/
- Cabiddu MF, Russi A, Appolloni L, Mengato D, Chiumente M. Omega-3 for the prevention of cardiovascular diseases: meta-analysis and trial-sequential analysis. Eur J Hosp Pharm. 2022 May;29(3):134-138. https://pubmed.ncbi.nlm.nih.gov/32546568/
- Selvaraj S, Bhatt DL, Steg PG, et al; REDUCE‐IT Investigators. Impact of Icosapent Ethyl on Cardiovascular Risk Reduction in Patients With Heart Failure in REDUCE-IT. J Am Heart Assoc. 2022 Apr 5;11(7):e024999. https://pubmed.ncbi.nlm.nih.gov/35377160/
- Peterson BE, Bhatt DL, Steg PG, et al; REDUCE‐IT Investigators. Treatment With Icosapent Ethyl to Reduce Ischemic Events in Patients With Prior Percutaneous Coronary Intervention: Insights From REDUCE-IT PCI. J Am Heart Assoc. 2022 Mar 15;11(6):e022937. https://pubmed.ncbi.nlm.nih.gov/35261279/
- Djoussé L, Cook NR, Kim E, et al. Diabetes Mellitus, Race, and Effects of Omega-3 Fatty Acids on Incidence of Heart Failure Hospitalization. JACC Heart Fail. 2022 Apr;10(4):227-234. https://pubmed.ncbi.nlm.nih.gov/35361440/
- Maki KC, Bays HE, Ballantyne CM, et al. A Head-to-Head Comparison of a Free Fatty Acid Formulation of Omega-3 Pentaenoic Acids Versus Icosapent Ethyl in Adults With Hypertriglyceridemia: The ENHANCE-IT Study. J Am Heart Assoc. 2022 Mar 15;11(6):e024176. https://pubmed.ncbi.nlm.nih.gov/35232215/
- De Cosmi V, Mazzocchi A, D'Oria V, et al. Effect of Vitamin D and Docosahexaenoic Acid Co-Supplementation on Vitamin D Status, Body Composition, and Metabolic Markers in Obese Children: A Randomized, Double Blind, Controlled Study. Nutrients. 2022 Mar 27;14(7):1397. https://pubmed.ncbi.nlm.nih.gov/35406010/
- Checa-Ros A, Haro-García A, Seiquer I, Molina-Carballo A, Uberos-Fernández J, Muñoz-Hoyos A. Early monitoring of fatty acid profile in children with attention deficit and/or hyperactivity disorder under treatment with omega-3 polyunsaturated fatty acids. Minerva Pediatr. 2019 Aug;71(4):313-325. https://pubmed.ncbi.nlm.nih.gov/30419741/
- Salehi B, Mohammadbeigi A, Sheykholeslam H, et al. Omega-3 and Zinc supplementation as complementary therapies in children with attention-deficit/hyperactivity disorder. J Res Pharm Pract. 2016 Jan-Mar;5(1):22-6. https://pubmed.ncbi.nlm.nih.gov/26985432/
- Barragán E, Breuer D, Döpfner M. Efficacy and Safety of Omega-3/6 Fatty Acids, Methylphenidate, and a Combined Treatment in Children With ADHD. J Atten Disord. 2017 Mar;21(5):433-441. https://pubmed.ncbi.nlm.nih.gov/24464327/
- Perera H, Jeewandara KC, Seneviratne S, Guruge C. Combined ω3 and ω6 supplementation in children with attention-deficit hyperactivity disorder (ADHD) refractory to methylphenidate treatment: a double-blind, placebo-controlled study. J Child Neurol. 2012 Jun;27(6):747-53. https://pubmed.ncbi.nlm.nih.gov/22596014/
- Bonvicini C, Faraone SV, Scassellati C. Attention-deficit hyperactivity disorder in adults: A systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studies. Mol Psychiatry. 2016 Jul;21(7):872-84. doi: 10.1038/mp.2016.74. https://pubmed.ncbi.nlm.nih.gov/27217152/







